Rapid onset opioids in palliative medicine
Introduction
Opioids are potent broad spectrum analgesics used for pain relief in a variety of painful conditions. Opioids/opioid formulations may be classified based on their pharmacokinetic characteristics (e.g., long-acting opioids, short-acting opioids, and rapid onset opioids). Long-acting opioids have been traditionally classified into two main groups: (I) pharmaceutically enhanced opioid analgesics and (II) pharmacologically long-acting agent comprising opioids with inherently long-acting pharmacokinetic profiles (e.g. methadone, levorphanol) (Table 1). Pharmaceutically-enhanced opioid analgesics are often referred to as controlled-release (CR) opioids, sustained-release (SR) opioids, or extended-release (ER) opioids.
Table 1
| Pharmacologically long-acting | Pharmaceutically long-acting |
|---|---|
| Levorphanol (Levo-Dromoran) | Hydromorphone (Exalgo) |
| Methadone (Dolophine) | Morphine sulfate (Kadian, AVINZA, MS Contin, Oramorph SR) |
| Oxycodone (Oxycontin) | |
| Oxymorphone (Opana ER) | |
| Transdermal fentanyl (3 day patch) (Duragesic) | |
| Transdermal Buprenorphine (7 day patch) (Butrans) | |
| Tramadol ER (Ultram ER, Ryzolt) | |
| Tapentadol ER (Nucynta) |
Short-acting opioids are the opioids analgesics often used for acute or subacute outpatient opioid therapy and include: codeine, hydrocodone, oxycodone, oxymorphone, and morphine. Short-acting opioids analgesics are often referred to immediate–release (IR) opioids [e.g., Morphine sulfate immediate release (MSIR), Oxycodone immediate release (OxyIR)].
Breakthrough pain and its treatment
Persistant pain in palliative medicine and in particular cancer-induced chronic pain, often does not exist as a single entity, but instead may be considered as a combination of background pain and breakthrough pain. Breakthrough pain (BTP) has been defined as 'a transitory exacerbation of pain experienced by the patient who has relatively stable and adequately controlled baseline pain' (1). Breakthrough pain can be divided into spontaneous pain at rest and incident pain (either volitional or non-volitional) (2,3). Breakthrough pain was present in 75% of cases of cancer-induced bone pain (4). Patients with breakthrough pain had greater interference on aspects of life (mood, relationships, sleep, activity, walking ability, work, enjoyment of life) than those with no breakthrough pain (P<0.01) (5,6). Almost half of breakthrough pain episodes were rapid in onset (<5 min) and short in duration (<15 min) (5,6). Forty-four per cent of patients with breakthrough pain had pain that was unpredictable (5,6). The short spiking characteristics of BTP episodes make the successful treatment of cancer-induced bone pain particularly challenging, which is supported by studies revealing that up to 45% of patients with cancer-induced bone pain report poor pain control (6-8).
Traditionally, background pain (experienced for over 12 hours/day) has been treated with long-acting opioids (Morphine Sulfate controlled-release formulation [e.g., MS contin]) and breakthrough pains have been treated with short-acting opioids (e.g., Morphine sulfate immediate-release [e.g., MSIR]). However, some breakthrough pain episodes occur without warning and reach peak severe intensity within 5 minutes. For the treatment of these painful breakthrough pain episodes, rapid onset opioids are required.
Rapid onset opioids
At present the only rapid-onset analgesic that is suitable for the treatment of rapid-onset breakthrough pain (ROBTP) is fentanyl, a µ-opioid receptor agonist with potent analgesic properties. Fentanyl is a synthetic phenylpiperidine that is roughly 80 times more potent than morphine. It is highly lipophilic and binds avidly to plasma proteins. Fentanyl has an n-octanol:water partition coefficient at pH 7.4 of 816:1 (8) versus 1.4 for morphine and 0.7 for oxycodone (9). The high octanol/water coefficients for fentanyl may explain why it is well absorbed across the oral mucosa and why it takes fentanyl shorter than morphine to oxycodone to move across the blood-brain barrier to enter the central nervous system (9).
Rapid-onset opioids FDA approved in the United States include: oral transmucosal fentanyl citrate (OTFC) [Atiq®], fentanyl buccal tablet (FBT) [Fentora®], fentanyl buccal soluble film (FBSF) [Onsolis®], sublingual fentanyl (SLF) [Abstral®], and fentanyl pectin nasal spray (FPNS) [Lazanda®] (Figure 1). Potential future rapid-onset opioids may include: intranasal fentanyl spray (INFS) [Instanyl®] and fentanyl dry powder intrapulmonary inhaler [TAIFUN®]. Clinicians should initiate therapy with the lowest dose available, 100 mcg if possible; and should attempt to only use a maximum of two doses per breakthrough pain episode and preferably no more than 4 breakthrough pain episodes per day.
Oral Transmucosal Fentanyl Citrate (OTFC)
OTFC (Actiq®) is a sweetened lozenge containing fentanyl citrate that is attached to a stick to help the patient sweep the medication across the buccal mucosa (lining of the cheek). Administration of the lozenge takes approximately 15 min (10). OTFC was approved in the USA in 1998 for BTP in adults with cancer who are receiving, and are tolerant of, opioid analgesics for underlying chronic cancer pain. OTFC was approved in Europe for the same indication in 2002. OTFC is available in six dose strengths - 200, 400, 600, 800, 1200, and 1600 µg lozenges. The oral mucosal route of delivery offers some advantages. The oral mucosa is highly permeable, 20 times more than skin; and highly vascularized.
Pharmacokinetics
When the OTFC lozenge is administered as directed, 25% of the total dose of fentanyl is absorbed by the buccal mucosa and becomes systemically available. Approximately 75% of the OTFC dose is swallowed and is then absorbed from the gastrointestinal tract where two-thirds is eliminated via first-pass metabolism (11). The bioavailability of OTFC is therefore approximately 50% of the total dose, split evenly between transmucosal and (slower) gastrointestinal absorption (11).
Clinical efficacy vs. Placebo
The efficacy of OTFC has been compared against placebo in a multicenter, double-blind, randomized study of opioid-tolerant patients with cancer and BTP (12). Compared with BTP episodes in patients administered placebo, PID scores for episodes in those treated with OTFC were significantly greater from 15 min to 1 h after administration (P<0.0001) (12). Significant differences between OTFC and placebo were also evident in terms of global performance (mean scores 1.98 and 1.19 for OTFC and placebo, respectively; P<0.0001) and use of rescue medications (supplementary medication taken in addition to the initial dose of opioid for BTP; 15% vs. 34% of episodes, respectively; P<0.0001).
Safety and Tolerability
Across the clinical studies of OTFC, reported adverse effects were typical of opioids and included somnolence, nausea, and dizziness (12-14). For example, in a placebo-controlled, randomized, double-blind study, the most common treatment-emergent adverse events were dizziness (17% of patients), nausea (14%), somnolence (8%), constipation (5%), asthenia (5%), confusion (4%), vomiting (3%), and pruritus (3%) (12). Hallucinations and confusion relating to the use of OTFC have also been reported in clinical studies of this formulation (15).
Fentanyl Buccal Tablet (FBT)
The FBT Fentora® was approved in the USA in 2006 for BTP in adults with cancer pain who are receiving and are tolerant of opioid analgesics for underlying chronic cancer pain. FBT is available in doses of 100, 200, 400, 600, and 800 µg buccal tabs. FBT uses OraVescent® delivery technology to alter the pH of the oral environment in order to assist with dissolution and maximize absorption of fentanyl. Dissolution takes 14-25 min with FBT and does not require active participation from the patient (16). The OraVescent® system produces an effervescence reaction that releases carbon dioxide to produce carbonic acid in the buccal cavity. The resultant decrease in pH optimizes tablet dissolution. FBT then releases sodium carbonate to increase the pH in order to increase permeation of fentanyl through the buccal mucosa (17,18). The buccal pH changes orchestrated by this effervescence reaction result in a greater proportion of fentanyl being absorbed transmucosally instead of being swallowed and absorbed by the slower gastrointestinal route. Furthermore, because 50% of the fentanyl in FBT is absorbed transmucosally (19), cytochrome P450 metabolism is bypassed to a greater extent than with traditional short-acting opioids and OTFC, so a greater proportion of fentanyl enters the systemic circulation (20).
Pharmacokinetics
After FBT administration, fentanyl was rapidly absorbed in a dose-dependent fashion, with Tmax ranging from 20 minutes to 4 hours postdose. Mean AUC(0-∞) was 1.49 ng•hour/mL, and mean Cmax was 0.237 ng/mL. However, plasma fentanyl concentration reached 80% of Cmax within 25 minutes and was maintained through 2 hours after administration (21).
In a study of 39 healthy volunteers that evaluated the single-dose pharmacokinetics of FBT (270-1,300 µg), mean t1/2 values ranged from 6.6 h to 13.2 h (20). A lower dose of FBT (1080 µg) provided comparable systemic exposure to that of a higher dose of OTFC (1600 µg) (20).
Clinical efficacy vs. Placebo
FBT has been shown to confer statistically and clinically significant improvements in the treatment of BTP in patients with cancer and noncancer pain in five placebo-controlled studies (22-26). In brief, compared with placebo, FBT demonstrated significant reductions in SPID60 and PID from 10 min, significant increases in pain relief from 10 min, lower rates of rescue medication use, significantly greater medication performance assessment scores, and moderate and substantial clinically relevant improvements in pain intensity from 5 and 15 min, respectively (22-26).
Safety and tolerability
No unexpected safety or tolerability concerns have been noted with FBT. The most common adverse events were nausea, dizziness, and vomiting (27). In an 18-month safety analysis that included 646 patients with chronic noncancer pain and BTP, patients were exposed to a median of 329 (range 1-638) days of treatment with FBT in order to treat a median of 1110 (range 1-5226) episodes of BTP (28). Adverse events experienced with FBT were typical of opioids and decreased in incidence over time.
Fentanyl buccal soluble film (FBSF)
The fentanyl buccal soluble film (FBSF) Onsolis™ utilizes BioErodible MucoAdhesive (BEMA™) technology (BioDelivery Sciences International). It was approved in the USA in 2009 for BTP in adults with cancer who are receiving and who are tolerant of opioid analgesics for chronic cancer pain. FBSF is available in doses of 200, 400, 600, 800, and 1200 µg per film.
FBSF presents fentanyl in a layer that adheres to the inside of the patient's cheek; an outer layer isolates the fentanyl-containing layer from saliva. In this way, the FBSF minimizes the quantity of fentanyl that is swallowed in the saliva and which is consequently lost during first-pass metabolism (29).
Pharmacokinetics
The absolute bioavailability of fentanyl from FBSF was reported to be 71%, with approximately 51% of the administered dose being absorbed through the buccal mucosa (29). FBSF demonstrated low intraindividual pharmacokinetic variability (coefficient of variation 7-10%) in a study of 24 healthy subjects, indicating that it would be expected to have consistent effects within a single individual in clinical practice (30).
Clinical efficacy vs. Placebo
The efficacy of FBSF has been assessed in a multicenter, randomized, placebo-controlled, multiple crossover study of 80 opioid-tolerant adult patients with cancer who experienced BTP. Patients were eligible to enter the double-blind crossover period if they were successfully titrated within a 2-week period to an FBSF dose (200-1200 µg) that provided suitable pain relief. Compared with placebo, FBSF significantly reduced pain intensity, as measured by the SPID at 30 min (SPID30; 38.1 vs. 47.9, respectively; P=0.004) (31). A statistically significant (P<0.05) improvement with FBSF over placebo was reported for the SPID from 15 min and persisted to the last time point assessed in this study (60 min; P<0.001) (31). PID over time was statistically significantly greater for FBSF vs. placebo from 30 min until the final assessment (P<0.01). The percentage of BTP episodes with a 33% [64.3% for FBSF vs. 48.2% for placebo (P<0.001)] or 50% [46.3% for FBSF vs. 34.0 for placebo (P<0.005)] decrease in pain scores 60 minutes after administration was significantly greater with FBSF than with placebo (31).
Safety and tolerability
Similar to the other fentanyl formulations described, FBSF has been reported to be well tolerated with an adverse-event profile typical of opioid analgesics. In a multicenter, placebo-controlled study of FBSF in opioid-tolerant patients with cancer and BTP, the most common drug-related adverse events were somnolence (6.0%), nausea (5.3%), dizziness (4.6%), and vomiting (4.0%). The rate of patient drop-out due to treatment-emergent adverse events was 13.9% in this study (31).
Sublingual fentanyl (SLF)
SLF (Abstral®) was approved in the USA for opioid-tolerant adults with cancer in 2011. The sublingual mucosa is highly vascularized and has good permeability, allowing rapid absorption of fentanyl (32). SLF is a tablet comprising water-soluble carrier particles that are coated with fentanyl and a mucoadhesive agent to hold the tablet under the tongue. SLF is available in doses of 100 µg, 200, 300, 400, 600, and to 800 µg sublingual tabs. The median dose used in a Phase III study of 60 patients with cancer and BTP was 600 µg (mean 550.8 µg) and a median of three doses were taken each day (33).
Pharmacokinetics
Total fentanyl exposure with SLF was proportional to the administered dose (dose range 100-400 µg) in a pharmacokinetics analysis of 11 patients with cancer (34). Systemic exposure and absorption increased in a linear fashion with the doses assessed, and dose proportionality was also reported for the Cmax of SLF (100 µg 0.24 ng/mL, 200 µg 0.41 ng/mL, and 400 µg 0.91 ng/mL). Tmax ranged from 40 to 60 minutes for the 100μg and 400 μg doses, respectively (34).
Clinical efficacy vs. Placebo
In a small crossover study of 27 adult patients with locally advanced cancer and BTP, patients received placebo and SLF 100, 200, or 400 µg for one BTP episode in a random order separated by a washout period of 1 day (32). This study did not use a preliminary titration phase to find the dose with optimum efficacy and minimal adverse events for each patient. SLF 400 µg was associated with the greatest improvements in PID when compared with placebo and the other doses assessed. SLF 400 µg demonstrated an improvement of 8.57 mm (on a 100 mm VAS) compared with placebo over the treatment period (P<0.0001) and also gave a clinically (>20 mm) and statistically significant improvement in PID at an earlier time point (15 min; P=0.005) compared with the other doses (32). Use of rescue medication was significantly less common with SLF 400 µg compared with placebo (5 vs. 15 patients, respectively; P=0.001).
Safety and tolerability
In a Phase II study of patients with cancer and BTP, one instance each of mild-to-moderate vomiting and dizziness was thought to be related to the use of SLF 400 µg; the occurrence of adverse events did not increase with increasing SLF dose (32). Approximately a third of patients with cancer and BTP (41/131 patients in the safety population) in a Phase III study of SLF experienced an adverse event that was considered to be related to study medication. The most common adverse events in this study were nausea (12.2%), vomiting (5.3%), and somnolence (4.6%) (33).
Fentanyl Pectin Nasal Spray (FPNS)
The fentanyl pectin nasal spray (FPNS), Lazanda® (US trade name), in the US in 2011 for BTP in adults with cancer who are receiving and who are tolerant of opioid analgesics for chronic cancer pain. The addition of pectin in FPNS promotes the formation of a gel on contact with calcium cations on the nasal mucosa, prolonging the residence time of fentanyl at the mucosa and giving a rounded pharmacokinetic profile compared with the sharp profile of non-gelling sprays (35,36). This pectin-based drug delivery system is referred to as PecSys (36). The high, early Cmax of the non-gelling sprays is reported to be indicative of a wide coefficient of variation and less predictable efficacy and tolerability (35). Furthermore, FPNS has demonstrated a slower decline in plasma fentanyl levels compared with non-gelling nasal sprays, suggesting that FPNS provides comparably extended analgesia vs. non-gelling intranasal formulations (35).
Pharmacokinetics
In a study of healthy volunteers, Tmax for FPNS was approximately 20 minutes and the Cmax was 337 pg/mL, with a t½ mean ranging from 15-24.9 hours (depending on dose (35).
Clinical efficacy vs. Placebo
In a randomized, placebo-controlled study of 83 opioid-tolerant patients with cancer and BTP, clinically relevant reductions of ≥2 points in absolute pain intensity (measured on an 11-point numeric scale) were observed within 10 min in 33% of BTP episodes treated with FPNS vs. 25% of patients given placebo (P<0.05). Clinically meaningful improvements in pain relief were also recorded at 10 min with FPNS (33% vs. 24% for placebo; P<0.01) (37). Rescue medication use was required within 60 min in 9% of BTP episodes treated with FPNS compared with 20% of episodes treated with placebo (P<0.001) (37). A number of additional endpoints were assessed in this study of FPNS and published in a separate paper (38). FPNS demonstrated significantly greater mean SPID30 scores compared with placebo (6.57 vs. 4.45; P<0.0001) (38). Compared with placebo, a significantly greater proportion of patients treated with FPNS reported onset of analgesia (≥1 point-reduction in pain intensity score) from 10 min (38.4% vs. 56.2%; P<0.01). Furthermore, the reduction in pain intensity became clinically meaningful (≥2 point-reduction) for 49% of FPNS-treated patients at 15 min and 63% at 30 min (38). Clinically meaningful pain relief was reported by a significantly higher proportion of patients receiving FPNS vs. placebo from 10 min (32.9 vs. 24.5; P=0.01). Rescue medication within 60 min was required during 9.4% of BTP episodes treated with FPNS compared with 20.0% of episodes treated with placebo (P<0.001) (38).
Safety and tolerability
Approximately half of patients receiving FPNS experienced treatment-emergent adverse events in a placebo-controlled, multiple-crossover (38). Nasal adverse events reported in a study (n=89) comparing FPNS with immediate-release morphine sulfate included mild obstruction (2.2%) and mild nasal discharge (4.5%) (39).
A useful clinical niche for rapid onset opioids
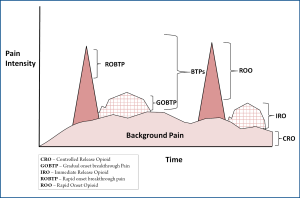
A particularly useful clinical niche for rapid onset opioids is for the treatment of rapid-onset breakthrough pain in advanced painful osseous metastases. In painful osseous metastases (POM), there are generally two types of breakthrough pain: gradual onset breakthrough pain (GOBTP) (usually coming on in a predictable fashion in 15-30 minutes and fading within an hour) and ROBTP (often unpredictable in nature may be paroxysmal and/or lacinating coming on and reaching a peak severe intensity within 5 minutes and fading within 15 minutes). These ROBTP episodes are extremely challenging to address and require treatment with rapid onset opioids.
Movement-evoked BTP in these patients is an especially difficult problem to address. Bäckryd and Larsson found that patients whose pain was well-controlled with intrathecal analgesia still had terrible suffering due to movement-evoked BTP (40). Brogan and Winter attempted to deal with breakthrough pain in patients receiving intrathecal analgesia by utilizing patient-controlled intrathecal analgesia (PCIA) and reported very preliminary retrospective beneficial results (41).
One of the major classes of agents for the pharmacologic management of POM is that of opioid analgesics. Preclinical research suggests that there may be varying efficacy for different opioids (42), however, clinically there does not appear to be any opioid that is better than any other opioid for the treatment of painful osseous metastases. Although some opioids may provide more analgesia than other opioids for a specific individual patient, currently, "trial and error" is the only way to determine this. Opioids are considered an effective therapy for background pain in POM, however, their usefulness in breakthrough pain is less clear. It appears to be vitally important to match the characteristics of the opioid utilized to treat BTP; to the type of BTP experienced. Immediate release oral morphine has, at best, an onset of action of about 30 min (43). This means that in patients with rapid-onset, short duration breakthrough pain, immediate release morphine will probably be ineffective. Furthermore, titration of opioids to doses that control episodes of breakthrough pain may result in unacceptable opioid side-effects (44). Newer, ultra-fast acting opioids have been developed with the aim of mirroring the temporal features of rapid onset breakthrough pain.
A "triple opioid therapy (TOT) approach" to using opioid analgesics may be optimal to treat painful osseous metastases. A triple opioid therapy approach utilizes three different opioid formulations (a controlled release opioid, an immediate release opioid, and an ultra-fast acting opioid). Enteral or transdermal extended release (ER) or controlled release (CR) opioids are employed for "maintenance" therapy to control the baseline or background constant pain. The patient receiving TOT then evaluates BTP episodes; (I) if a BTP episode seems relatively predictable and gradually intensifies over a half-hour or more [Gradual Onset Breakthrough Pain (GOBTP)], then it may be treated early with an immediate release (IR) opioid formulation, however, (II) if a BTP episode is unpredictable and/or the intensity suddenly increases rapidly [ROBTP], then it should be treated with a rapid onset opioid (Figure 2).
Summary
It is vitally important in palliative medicine, when treating the subpopulation of patients with advanced painful osseous metastases who have rapid onset breakthrough pain (ROBTP), to use analgesics with pharmacokinetic characteristics which match the temporal characteristics of the patient's ROBTP. ROBTP (which may occur spontaneously but is also often movement-induced may be unpredictable, may peak to severe intensities within five minutes or less and fade after 15 minutes) is likely suboptimally treated with short-acting opioids. Short-acting opioids have not even peaked by 15 minutes and are thus, doomed to provide ineffective analgesia for these types of ROBTP. Rapid onset opioids are often in a more favorable "pharmacokinetic" position to address the acute nature of these ROBTPs than traditional short-acting opioids.
There have been multiple fentanyl-based rapid onset opioid formulations introduced within the last five years, with two approved by the U.S. FDA in 2011. Currently, it is not possible to conclude that any formulation is superior to another, based on the paucity of well-designed head to head trials. Based on available data, largely from placebo-controlled trials; the current formulations appear relatively comparable in terms of safety and efficacy.
A key consideration in selecting specific rapid onset opioids is the need to match the specific rapid onset formulation to the individual patients and the individual patient's pain characteristics (including disease characteristics, patient attributes and preferences, ease of administration), More research needs to be done with existing agents as well as the development of future novel rapid onset opioids and other rapid onset analgesics.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
Footnote
No potential conflict of interest.
References
- Portenoy RK, Forbes K, Lussier D, et al. Difficult pain problems: an integrated approach. In: Doyle D, Hanks G, Cherny NI, Calman K editors. Oxford textbook of palliative medicine. Oxford : Oxford University Press;2004:438-58.
- Colvin L, Fallon M. Challenges in cancer pain management--bone pain. Eur J Cancer 2008;44:1083-90.
- Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev 1998;24:425-32.
- Laird BJ, Walley J, Murray G, et al. What is the key question in the assessment of cancer induced bone pain: results from a characterization study. London: British Pain Society Annual Scientific Meeting,2009.
- Laird BJ, Walley J, Murray GD, et al. Characterization of cancer-induced bone pain: an exploratory study. Support Care Cancer 2011;19:1393-401.
- Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 2011;23:387-92.
- de Wit R, van Dam F, Loonstra S, et al. The Amsterdam Pain Management Index compared to eight frequently used outcome measures to evaluate the adequacy of pain treatment in cancer patients with chronic pain. Pain 2001;91:339-49.
- Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247-57.
- Aronoff GM, Brennan MJ, Pritchard DD, et al. Evidence-based oral transmucosal fentanyl citrate (OTFC) dosing guidelines. Pain Med 2005;6:305-14.
- Actiq® Package Insert. Actiq® (oral transmucosal fentanyl citrate) Package Insert. Salt Lake City, UT: Cephalon, Inc.;2009.
- Streisand JB, Varvel JR, Stanski DR, et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 1991;75:223-9.
- Farrar JT, Cleary J, Rauck R, et al. Oral transmucosal fentanyl citrate: randomized, double-blinded, placebo-controlled trial for treatment of breakthrough pain in cancer patients. J Natl Cancer Inst 1998;90:611-6.
- Christie JM, Simmonds M, Patt R, et al. Dose-titration, multicenter study of oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients using transdermal fentanyl for persistent pain. J Clin Oncol 1998;16:3238-45.
- Portenoy RK, Payne R, Coluzzi P, et al. Oral transmucosal fentanyl citrate (OTFC) for the treatment of breakthrough pain in cancer patients: a controlled dose titration study. Pain 1999;79:303-12.
- Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR). Pain 2001;91:123-30.
- Fentora® Package Insert. Fentora® (fentanyl buccal tablet) Package Insert. Salt Lake City, UT: Cephalon, Inc.;2011.
- Durfee S, Messina J, Khankari R. Fentanyl effervescent buccal tablets: enhanced buccal absorption. Am J Drug Deliv 2006;4:1-5.
- Pather SI, Siebert JM, Hontz J, et al. Enhanced buccal delivery of fentanyl using the oravescent drug delivery system. Drug Deliv Technol 2001;1:54-57.
- Darwish M, Kirby M, Robertson P Jr, et al. Absolute and relative bioavailability of fentanyl buccal tablet and oral transmucosal fentanyl citrate. J Clin Pharmacol 2007;47:343-50.
- Darwish M, Tempero K, Kirby M, et al. Relative bioavailability of the fentanyl effervescent buccal tablet (FEBT) 1,080 pg versus oral transmucosal fentanyl citrate 1,600 pg and dose proportionality of FEBT 270 to 1,300 microg: a single-dose, randomized, open-label, three-period study in healthy adult volunteers. Clin Ther 2006;28:715-24.
- Darwish M, Xie F. Pharmacokinetics of Fentanyl Buccal Tablet: A Pooled Analysis and Review. Pain Pract 2011; [Epub ahead of print].
- Farrar JT, Messina J, Xie F, et al. A novel 12-week study, with three randomized, double-blind placebo-controlled periods to evaluate fentanyl buccal tablets for the relief of breakthrough pain in opioid-tolerant patients with noncancer-related chronic pain. Pain Med 2010;11:1313-27.
- Portenoy RK, Taylor D, Messina J, et al. A randomized, placebo-controlled study of fentanyl buccal tablet for breakthrough pain in opioid-treated patients with cancer. Clin J Pain 2006;22:805-11.
- Portenoy RK, Messina J, Xie F, et al. Fentanyl buccal tablet (FBT) for relief of breakthrough pain in opioid-treated patients with chronic low back pain: a randomized, placebo-controlled study. Curr Med Res Opin 2007;23:223-33.
- Simpson DM, Messina J, Xie F, et al. Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther 2007;29:588-601.
- Slatkin NE, Xie F, Messina J, et al. Fentanyl buccal tablet for relief of breakthrough pain in opioid-tolerant patients with cancer-related chronic pain. J Support Oncol 2007;5:327-34.
- Weinstein SM, Messina J, Xie F. Fentanyl buccal tablet for the treatment of breakthrough pain in opioid-tolerant patients with chronic cancer pain: A long-term, open-label safety study. Cancer 2009;115:2571-9.
- Fine PG, Messina J, Xie F, et al. Long-term safety and tolerability of fentanyl buccal tablet for the treatment of breakthrough pain in opioid-tolerant patients with chronic pain: an 18-month study. J Pain Symptom Manage 2010;40:747-60.
- Vasisht N, Gever LN, Tagarro I, et al. Single-dose pharmacokinetics of fentanyl buccal soluble film. Pain Med 2010;11:1017-23.
- Davies A, Finn A, Tagarro I. Intra- and interindividual variabilities in the pharmacokinetics of fentanyl buccal soluble film in healthy subjects: a cross-study analysis. Clin Drug Investig 2011;31:317-24.
- Rauck R, North J, Gever LN, et al. Fentanyl buccal soluble film (FBSF) for breakthrough pain in patients with cancer: a randomized, double-blind, placebo-controlled study. Ann Oncol 2010;21:1308-14.
- Lennernäs B, Frank-Lissbrant I, Lennernäs H, et al. Sublingual administration of fentanyl to cancer patients is an effective treatment for breakthrough pain: results from a randomized phase II study. Palliat Med 2010;24:286-93.
- Rauck RL, Tark M, Reyes E, et al. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin 2009;25:2877-85.
- Lennernäs B, Hedner T, Holmberg M, et al. Pharmacokinetics and tolerability of different doses of fentanyl following sublingual administration of a rapidly dissolving tablet to cancer patients: a new approach to treatment of incident pain. Br J Clin Pharmacol 2005;59:249-53.
- Fisher A, Watling M, Smith A, et al. Pharmacokinetics and relative bioavailability of fentanyl pectin nasal spray 100 - 800 µg in healthy volunteers. Int J Clin Pharmacol Ther 2010;48:860-7.
- Watts P, Smith A. PecSys: in situ gelling system for optimised nasal drug delivery. Expert Opin Drug Deliv 2009;6:543-52.
- Taylor D, Galan V, Weinstein SM, et al. Fentanyl pectin nasal spray in breakthrough cancer pain. J Support Oncol 2010;8:184-90.
- Portenoy RK, Burton AW, Gabrail N, et al. A multicenter, placebo-controlled, double-blind, multiple-crossover study of Fentanyl Pectin Nasal Spray (FPNS) in the treatment of breakthrough cancer pain. Pain 2010;151:617-24.
- Davies A, Sitte T, Elsner F, et al. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage 2011;41:358-66.
- Bäckryd E, Larsson B. Movement-evoked breakthrough cancer pain despite intrathecal analgesia: a prospective series. Acta Anaesthesiol Scand 2011;55:1139-46.
- Brogan SE, Winter NB. Patient-controlled intrathecal analgesia for the management of breakthrough cancer pain: a retrospective review and commentary. Pain Med 2011;12:1758-68.
- Kato A, Minami K, Ito H, et al. Oxycodone-induced analgesic effects in a bone cancer pain model in mice. Oncology 2008;74:55-60.
- Bailey F, Farley A. Oral opioid drugs. In: Davies A, editor. Cancer-related breakthrough pain. Oxford : Oxford University Press;2006:43-55.
- Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 1999;81:129-34.