Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting
Abstract: Chemotherapy can be a life-prolonging treatment for many cancer patients, but it is often
associated with profound nausea and vomiting that is so distressing that patients may delay or decline
treatment to avoid these side effects. The discovery of several NK1 receptor antagonists is a big revolution to
dealt this problem.
NK1 receptor antagonists prevent both acute and delayed chemotherapy-induced nausea and vomiting
(CINV).
These agents act centrally at NK-1 receptors in vomiting centers within the central nervous system to
block their activation by substance P released as an unwanted consequence of chemotherapy. By controlling
nausea and vomiting, these agents help improve patients’ daily living and their ability to complete multiple
cycles of chemotherapy. They are effective for both moderately and highly emetogenic chemotherapy
regimens. Their use might be associated with increased infection rates; however, additional appraisal of
specific data from RCTs is needed.
Key words: Chemotherapy; chemotherapy-induced nausea and vomiting; NK1 receptor
Introduction
Nausea and vomiting are common and feared symptoms among cancer patients (1-3), and up to 80% of patients will experience chemotherapy-induced nausea and vomiting (CINV) without prophylactic therapy (1-5). Nausea and vomiting can lead to deteriorated nutritional status, compromise adherence to therapy, and impair quality of life irrespective of etiology. Furthermore, inadequate emesis control may lead to anticipatory nausea and vomiting, which is a challenging clinical condition to treat and potentially refractory to standard medications (6,7).
The intrinsic risk of the chemotherapy regimen is the main risk factor for the overall degree of CINV and can vary depending on the class of drug, dose, schedule, and route of administration used. The current classification of the risk of emesis is mostly based on the intrinsic emetogenic potential of the chemotherapy regimen (8-10) which is stratified as follows: high emetogenic potential (>90% risk of inducing vomiting after chemotherapy administration), moderate emetogenic potential (>30-90% risk), low emetogenic potential (10-30% risk), and minimal emetogenic potential (<10% risk) (8).
Patient characteristics such as young age, female sex, low alcohol intake, poor performance status, previous history of bowel obstruction, history of motion sickness, and experience of emesis during pregnancy (11-13) may further increase the emetic risk but are currently not factors that are integrated into the choice of optimal prophylactic therapy. Additionally, disease-related features such as the primary site of the cancer, the histological subtype, clinical stage, presence of brain metastases, and presence of end organ dysfunction may further impact the probability of emesis. The use of adjunct therapies such opioid-derivatives, radiotherapy, or other medications can also exacerbate symptoms. Although the stratification usually applied to evaluate emetic risk does not consider important and relevant clinical and disease factors, it is accepted worldwide.
Cisplatin is the main example of a drug with a high emetogenic potential; doses greater than 50-mg/m2 lead to nausea and vomiting in more than 90% of patients if no prophylactic therapy is used (8). Other drugs with high emetogenic potential include cyclohosphamide (>1,500 mg/m2), carmustine (>250 mg/m2), and dacarbazine.
Efforts to prevent and treat CINV have been usually directed at blocking neurotransmitter receptors in the area postrema, which is a chemoreceptor trigger site for vomiting in response to emetic drugs. Dopamine, endorphin, serotonin, and neurokinin receptors are found in this area and are targets for preventing and treating CINV (14). Although the combination of dexamethasone and serotonin (5-HT3) receptor antagonists remained the backbone of CINV prevention until recently, this combination has been reported to lack effectiveness in preventing late onset CINV (15-20).
More than a decade ago, Navari et al. showed that neurokinin-1 receptor (NK1R) antagonists improve CINV when used in combination with cisplatin-based chemotherapy (21). These antagonists prevent the binding of substance P to the NK1R. Unopposed, substance P, a tachykinin family neuropeptide that functions as a neurotransmitter and neuromodulator, can mediate the induction of vomiting pathways by binding to the NK1R (22).
Current guidelines for CINV management (11,13,23) strongly recommend the use of NK1R antagonists for CINV prophylaxis in the acute and delayed phases for highly emetogenic chemotherapy schedules.
This review evaluates the overall effectiveness and safety of NK1R antagonists in the prevention of CINV (24).
History of the development
The substance P story has been nearly 80 years in the telling from the isolation of the peptide, to it’s sequencing, to the cloning of its receptor and the synthesis of the first small molecule antagonists.
The journey began in 1931 when a pharmacologically active substance was isolated from the brain and intestine and named substance P—“P” for powder—by the pioneering pharmacologist John Gaddum working with Ulf von Euler (25). In 1954, Gaddum continued his research into this extract and showed that substance P was concentrated in the emetic centers of the brain, commenting in his manuscript in the Journal of Physiology that “it is tempting to speculate why this is so” (26).
It was not until 40 years later, in 1971, that the active substance, an 11-amino acid peptide, was isolated, sequenced, and synthesized de novo by Susan Lehman and her group (27,28). Yet another 20 years went by, to 1991, before the receptor for substance P, the NK-1 receptor, was identified and cloned (29), and small-molecule blockers or antagonists of substance P that could access these NK-1 receptors in the emetic centers in the brain were developed. Amazingly, in line with Gaddum’s speculation, these substance P antagonists (SPAs) were shown to be the broadest spectrum antiemetics ever described.
Research at Merck began during the 1980s to discover SPAs with which to understand the role of substance P in health and disease. For many years this proved a difficult task, and only peptide antagonists that were unsuitable, as oral drug candidates and that did not cross the blood-brain barrier were synthesized.
In the early 1990s, the first small molecule brain-penetrant SPAs became available (30), enabling the investigation of the therapeutic potential of the NK-1 antagonist mechanism. Despite strong anatomical evidence supporting a potential role for substance P in pain and affective disorders, the SPAs were inactive as analgesics and as antidepressants. The hypothesis that substance P was involved in emesis was initially supported by three preclinical observations: substance P was localized in the emetic centers of the brain, substance P could cause emesis, and depletion of substance P using a toxin (resiniferatoxin) could prevent emesis in preclinical species with a vomiting reflex. The critical proof that the substance P/NK-1 receptor axis played a crucial role in mediating the vomiting response to a number of stimuli came with demonstration that highly selective SPAs had profound activity against emesis induced by broad range of central and peripherally acting emetogens (30,31). Moreover, these SPAs were active in multiple species with a vomiting reflex against a broad range of emetogens (32), giving high confidence that the mechanism would translate to clinically meaningful activity (33). In particular, SPAs were active against emesis induced by chemotherapeutic agents such as cisplatin and uniquely against the delayed phase of emesis that can recur days after treatment with such cytotoxic agents (34).
Continued research into the antiemetic mechanism of action of the SPAs showed that SPA molecules had to penetrate the brain to access central NK-1 receptors in the brain-stem emetic centers in order to be effective in preclinical models. Potent SPAs that did not cross into the brain were ineffective as antiemetics (35).
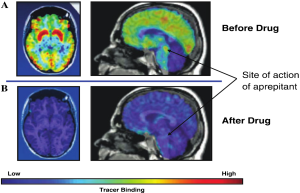
PET imaging of NK1-receptors in brain
Confirmation that drugs reach their targets using markers of engagement is key to successful proof- of-concept testing, especially for drugs acting in the brain. Knowing how hard and how long a drug must hit its target to produce the desired pharmacologic effect is important for dose selection and clinical trial design and interpretation.
A novel PET tracer that imaged NK-1 receptors in living human brain was developed to visualize the central sites of action of NK1-antagonist and to assess their occupancy by therapeutic doses of the drug (Figure 1). Understanding the relationships between dose, plasma concentration, and receptor occupancy for NK1 antagonist helped establish the link between target engagement and changes in the CINV clinical endpoint. This knowledge was important in the selection of the dosing regime for the regulatory filing these agents and ensured that the lowest drug exposure that achieved target engagement consistent with >90% blockade of NK-1 receptors was chosen, thereby maximizing the potential therapeutic safety window (36,37).
NK1 receptors antagonist: leraning about the biology of chemotherpay induced nausea
The development of multiple NK1 Receptors antagonists provided new insights into the pharmacological and pathophysiological mechanisms involved in emesis.
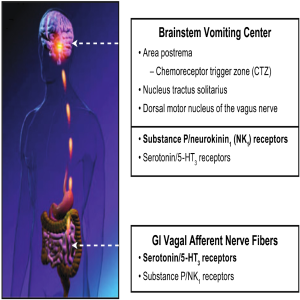
Both peripheral (glosophararyngeal and vagal nerves) and central (cortical and cerebellar) pathways can activate neuronal nuclei in the brainstem and trigger a sequence of events that results in the vomiting reflex. The 5-HT3 receptor antagonists are thought to exert their actions predominantly on the peripheral terminals of vagal afferents in the gastrointestinal tract and in the chemoreceptor trigger zone (CRTZ) that lays in the area postrema outside of the blood-brain barrier to block the activating effects of serotonin released during chemotherapy. The CRTZ signals to another area, the nucleus tractus solitarius (NTS), in the brain stem that also receives emetogenic stimuli from higher brain centers (e.g., cortical and vestibular) as well as gastrointestinal vagal afferents, and is thought to define the patterns of central activity underlying CINV.
Preclinical studies suggest that it is here within the NTS that NK1 receptors antagonists exert their strongest antiemetic properties through central inhibition of the emesis pattern generator. This central site of action is the likely explanation for the unique broad antiemetic pharmacological profile of these agents, indicating that substance P acting at central NK-1 receptors is one of the final common mechanisms involved in activation and coordination of the vomiting reflex (38).
The acute and delayed clinical time course of CINV has previously been linked to serotonin release and inflammation by the clinical effectiveness of 5-HT3 antagonists and steroids, respectively. The discovery of the NK 1 receptor antagonists and clinical experience with these agents has furthered our understanding and provided substantial evidence for involvement of substance P throughout the CINV response.
The prolonged efficacy profile of many of these agents, including the delayed phase, indicates that substance P acting at central NK-1 receptors becomes increasingly important with time in the pathophysiology of the overall CINV response (39).
These observations support the clinical rationale for combination therapy with 5-HT3 receptor antagonists (e.g., ondansetron), steroids (e.g., dexamethasone), and NK1 receptors antagonists to optimize control of CINV (Figure 2).
NK1 receptors antagonists and interactions with chemo-based agents
NK1R antagonists are known to increase the bioavailability of dexamethasone, and this pharmacokinetic interaction could potentially play a role in the higher incidence of infection among patients who have been treated with NK1R antagonists. The Chawla et al. study (40) did not decrease the day 1 dexamethasone dose in the NK1R arm, whereas the Schmoll et al. (41) and Poli-Bigelli et al. (42) studies did. Nevertheless, it seems unlikely that increased dexamethasone bioavailability could have any impact on the infection rates because these three trials presented similar findings.
NK1R antagonists can also increase the bioavailability of chemotherapy agents metabolized by cytochrome P450 3A4 (CYP3A4), such as etoposide, taxanes, irinotecan, vinca alkaloids, anthracyclines, and cyclophosphamide. Two of three studies suggested that adverse events could be more common among patients receiving an NK1R antagonist plus a CYP3A4-metabolized chemotherapy (43).
Current and future challenges
Ongoing discovery and development efforts in the program aim to identify simplified dose regimens of these newly made NK 1 receptors agents.
In August 2010, a single-dose formulation of fosaprepitant (NK 1 Receptor antagonist) for IV administration was approved in the European Union for use instead of the 3-day oral regimen of aprepitant (another NK 1 receptor antagonist), together with a 5-HT3 receptor antagonist and a corticosteroid.
Additionally, in November 2010, the FDA approved a single-dose formulation, EMEND for Injection 150 mg, for patients receiving highly emetic chemotherapy. This new regimen provides a more simple treatment to patients who will not need to worry about taking capsules of EMEND on days two and three, ensuring compliance with therapy and improving convenience for both patients and healthcare professionals.
The incidence of cancer in pediatric patients is still a concern despite the availability of treatments for the different tumors and the resilience of the patients. There are no clear guidelines for antiemetic therapy in pediatric cancer patients since the available scientific data are very limited; this allows pediatric cancer patients to go through their chemotherapy treatment with suboptimal therapy for prevention of CINV. An age-appropriate formulation of these agents (powder for suspension) is currently under research and development to extend its benefits to pediatric patients.
NK1 receptors antagonists and infections
However, the increased risk of severe infection is probably not explained by hematological toxicity because leukopenia, neutropenia, severe neutropenia, and febrile neutropenia were not increased by the addition of NK1R antagonists.
NK1R is known to have a role in neurogenic response to injury, and its suppression might impair natural defenses against infection (44-46) that might predispose patients to a greater risk of infection through immune-mediated mechanisms that are poorly understood.
Conclusions
In conclusion, NK1R antagonists improved control of CINV in the acute, delayed, and overall phases for patients who received highly and moderately emetogenic chemotherapy. CINV control in the acute phase seemed to be a surrogate for CINV control in the delayed phase. The use of NK1R antagonists may be associated with a statistically significantly increased risk of severe infection. A more comprehensive evaluation of the safety profile of NK1R antagonists and additional appraisal of specific data from RCTs is needed.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Laszlo J, Lucas VS Jr. Emesis as a critical problem in chemotherapy. N Engl J Med 1981;305:948-9.
- Morran C, Smith DC, Anderson DA, et al. Incidence of nausea and vomiting with cytotoxic chemotherapy: a prospective randomised trial of antiemetics. Br Med J 1979;1:1323-4.
- Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 2007;34:94-104.
- Griffin AM, Butow PN, Coates AS, et al. On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 1996;7:189-95.
- Osoba D, Zee B, Warr D, et al. Quality of life studies in chemotherapy-induced emesis. Oncology 1996;53:92-5.
- Wilcox PM, Fetting JH, Nettesheim KM, et al. Anticipatory vomiting in women receiving cyclophosphamide, methotrexate, and 5-FU (CMF) adjuvant chemotherapy for breast carcinoma. Cancer Treat Rep 1982;66:1601-4.
- Morrow GR, Lindke J, Black PM. Predicting development of anticipatory nausea in cancer patients: prospective examination of eight clinical characteristics. J Pain Symptom Manage 1991;6:215-23.
- Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;15:103-9.
- Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 1999;17:2971-94.
- Prevention of chemotherapy- and radiotherapy-induced emesis: results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Ann Oncol 1998;9:811-9.
- American Society of Clinical Oncology, Kris MG, Hesketh PJ, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 2006;24:2932-47.
- Roila F, Donati D, Tamberi S, et al. Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer 2002;10:88-95.
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Antiemesis Version 1. 2012. Fort Washington, PA: NCCN, 2011.
- Borison HL, McCarthy LE. Neuropharmacology of chemotherapy-induced emesis. Drugs 1983;25:8-17.
- Jantunen IT, Kataja VV, Muhonen TT. An overview of randomised studies comparing 5-HT3 receptor antagonists to conventional anti-emetics in the prophylaxis of acute chemotherapy-induced vomiting. Eur J Cancer 1997;33:66-74.
- Smith DB, Newlands ES, Rustin GJ, et al. Comparison of ondansetron and ondansetron plus dexamethasone as antiemetic prophylaxis during cisplatin-containing chemotherapy. Lancet 1991;338:487-90.
- Roila F, Tonato M, Cognetti F, et al. Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol 1991;9:675-8.
- Hesketh PJ, Harvey WH, Harker WG, et al. A randomized, double-blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high-dose cisplatin-induced emesis. J Clin Oncol 1994;12:596-600.
- Hesketh P. Management of cisplatin-induced delayed emesis. Oncology 1996;53:73-7.
- Roila F, Bracarda S, Tonato M, et al. Ondansetron (GR38032) in the prophylaxis of acute and delayed cisplatin-induced emesis. Clin Oncol (R Coll Radiol) 1990;2:268-72.
- Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med 1999;340:190-5.
- Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci 2003;91:87-94.
- Herrstedt J, Roila F, ESMO Guidelines Working Group. Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol 2008;19:ii110-2.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.
- V Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol 1931;72:74-87.
- Amin AH, Crawford TB, Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol 1954;126:596-618.
- Chang MM, Leeman SE, Niall HD. Amino acid sequence of substance P. Nat New Biol 1971;232:86-7.
- Tregear GW, Niall HD, Potts JT Jr, et al. Synthesis of substance P. Nat New Biol 1971;232:87-9.
- Fong TM, Anderson SA, Yu H, et al. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol 1992;41:24-30.
- Snider RM, Constantine JW, Lowe JA 3rd, et al. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science 1991;251:435-7.
- Bountra C, Bunce K, Dale T, et al. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 1993;249:R3-4.
- Tattersall FD, Rycroft W, Hargreaves RJ, et al. The tachykinin NK1 receptor antagonist CP-99,994 attenuates cisplatin induced emesis in the ferret. Eur J Pharmacol 1993;250:R5-6.
- Watson JW, Gonsalves SF, Fossa AA, et al. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol 1995;115:84-94.
- Andrews PLR, Rudd JA. The role of tachykinins and the tachykinin NK1 receptor in nausea and emesis. In: Holzer P. eds. Handbook of Experimental Pharmacology. New York: Springer, 2004:359-440.
- Tattersall FD, Rycroft W, Cumberbatch M, et al. The novel NK1 receptor antagonist MK-0869 (L-754, 030) and its water soluble phosphoryl prodrug, L-758,298, inhibit acute and delayed cisplatin-induced emesis in ferrets. Neuropharmacology 2000;39:652-63.
- Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov 2003;2:566-80.
- Bergström M, Hargreaves RJ, Burns HD, et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol Psychiatry 2004;55:1007-12.
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med 2001;111:106S-112S.
- Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 2003;39:1074-80.
- Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 2003;97:2290-300.
- Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 2006;17:1000-6.
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090-8.
- Grunberg SM, Rolski J, Strausz J, et al. Efficacy and safety of casopitant mesylate, a neurokinin 1 (NK1)-receptor antagonist, in prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:549-58.
- Beer S, Weighardt H, Emmanuilidis K, et al. Systemic neuropeptide levels as predictive indicators for lethal outcome in patients with postoperative sepsis. Crit Care Med 2002;30:1794-8.
- Chavolla-Calderón M, Bayer MK, Fontán JJ. Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation. J Clin Invest 2003;111:973-80.
- Verdrengh M, Tarkowski A. The impact of substance P signalling on the development of experimental staphylococcal sepsis and arthritis. Scand J Immunol 2008;67:253-9.