Palliative care for patients with locally advanced and metastatic non-small cell lung cancer
Introduction
Approximately 1.6 million people worldwide are diagnosed with lung cancer annually, of which about 85% of cases are non-small cell lung cancer (NSCLC). With nearly 1.4 million deaths annually, lung cancer is the leading cause of deaths attributable to malignancies (1). Locally advanced NSCLC patients have a median survival of 10-17 months, whereas patients with metastatic disease have a median survival of only 6-9 months (2). Patients with advanced disease often have significant symptoms and reduced quality of life (3,4). Palliative care, therefore, plays a prominent role in the care and management of patients with advanced NSCLC. Multiple studies have shown that poor quality of life correlates with shorter survival times among patients with metastatic NSCLC (5,6). Despite these findings, palliative care is often administered late in the disease course (7,8), a time when intervention is less able to improve patient outcomes or quality of life (9).
This review details the role of palliative and supportive care for patients with advanced and metastatic NSCLC and assesses palliative care broadly, as well as specific interventions meant to improve patient quality of life and clinical outcomes. The use of systemic therapy, radiation therapy, endobronchial therapies, and surgery as interventional palliative measures to improve symptoms for patients with NSCLC are also described.
Early palliative care, symptoms, and overall survival
Palliative care is defined by the World Health Organization as an approach to improve the quality of life for patients and their families who face life-threatening illnesses through the prevention and relief of suffering by early identification, impeccable assessment, and treatment of pain and other physical, psychosocial, and spiritual problems (10). Palliative care focuses on symptomatic management and aids patients and their caregivers with psychosocial support and decision making (11,12). Interdisciplinary palliative care teams use open communication to focus on patient symptoms and patient and family coping and quality of life in a broad context. Palliative care teams frequently include a combination of physicians, nurses, social workers, nutritionists, physical therapists, occupational therapists, and chaplains.
Palliative care can be administered concurrently with standard NSCLC treatment modalities used to attempt to prolong life, but the role of palliative care in this capacity has often been limited by physician perceptions that palliative care is an alternative to life-prolonging anti-cancer interventions. As a result, palliative interventions are often initiated when standard NSCLC treatment modalities are no longer deemed beneficial or tolerable (13). Combining palliative care with standard NSCLC-directed therapies can improved quality of life and patient symptoms and may also lead to improvements in overall survival (14).
In one study in the New England Journal of Medicine of 151 patients with newly diagnosed metastatic NSCLC who were randomized to receive early palliative care with standard oncologic care or standard oncologic care alone, early palliative care improved patient quality of life and mood, reduced rates of depressive symptoms at 12 weeks following randomization (16% vs. 38%, P=0.01), and prolonged the median survival (11.6 vs. 8.9 months, P=0.02) (15). Similarly, a review of United States Medicare beneficiary claims of 700 patients with lung cancer who enrolled in hospices and 586 unmatched non-hospice patients with lung cancer of similar age, sex, and race demonstrated that hospice care significantly prolonged the mean survival (279 vs. 240 days, P<0.0001) (16).
In another randomized study that included 117 patients with advanced lung cancer, nurse-lead psychoeducational intervention administered concurrently with cancer therapy significantly improved quality of life and reduced the occurrence and duration of depressed mood (both P=0.02), while also numerically increasing overall survival (14.0 vs. 8.5 months, P=0.14) (17). Studies from multi-institutional oncology collaborative groups, including the European Organisation for Research and Treatment of Cancer (18) and the Radiation Therapy Oncology Group (5), have also demonstrated that quality of life significantly influences survival in patients with NSCLC.
Palliative care in advanced NSCLC
Based on the improvements in quality of life and survival with early integration of palliative care in metastatic NSCLC, several national organizations including the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) now recommend the early integration of palliative care for patients with advanced NSCLC (19,20). However, the question arises as to what specifically palliative care offers to patients with advanced NSCLC.
Several descriptions of the fundamental components of a palliative care program exist. The National Consensus Project for Quality Palliative Care initially created a set of Clinical Practice Guidelines in 2004 and updated the Clinical Practice Guidelines in 2013. These guidelines broadly recommend the interdisciplinary palliative care team focus on the patient and their family across eight domains of care that include: (I) the structure and processes of care, concentrating on coordination of care across healthcare settings; (II) the physical aspects of care, concentrating on intensive symptom management; (III) the psychological and psychiatric aspects of care, concentrating on diagnosis and management of psychological distress as well as bereavement; (IV) the social aspects of care, concentrating on patient and family strengths and challenges; (V) the spiritual, religious and existential aspects of care, concentrating on general and specialized spiritual care; (VI) the cultural aspects of care, concentrating on cultural competency as integral to the care of patient and family; (VII) the care of the patient at the end of life, and (VIII) the ethical and legal aspects of care (21). With such broadly defined foci for palliative care, it becomes a challenge to determine if specific aspects of palliative care lead to the improved symptom management, improved quality of life, and lengthened overall survival that has been demonstrated in patients with advanced and metastatic NSCLC receiving early administration of palliative care integrated with standard oncologic care.
Pre-planned descriptions and retrospective chart reviews have described the elements of the palliative care interventions used in the New England Journal of Medicine trial that demonstrated benefits to early integration of palliative care (22,23). In an analysis of the initial clinical encounters with the palliative care team, Jacobsen et al. found the median time for the entire encounter was 55 minutes, with a median of 20 minutes devoted to symptom management, a median of 15 minutes devoted to assessment of patient and family coping, a median of 10 minutes devoted to illness understanding, and the remainder of the encounter generally divided between decision-making preferences and planning/referrals (22). Patients were often open to discussions of prognosis, even at the initial encounter. Conversations about advanced care planning were often deferred to later visits.
In a separate chart review of clinical visits of 20 of the New England Journal of Medicine early integration of palliative care study patients, Yoong et al. found seven key elements of the palliative care visits that included: (I) relationship and rapport building; (II) addressing symptoms; (III) addressing coping; (IV) establishing illness understanding; (V) discussing cancer treatments; (VI) end-of-life planning, and (VII) engaging family members. Trends in visit content indicated that relationship and rapport-building, assessment of prognostic awareness, and information preferences were prominent in early visits and decreased over time, whereas end-of-life planning and decision-making regarding cancer-directed therapy were less prominent in early visits and increased in later visits. Symptom management, coping, and family engagement were prominent at visits throughout the course of illness (23).
Physical symptoms of advanced NSCLC
Patients with advanced and metastatic NSCLC often present with pain, dyspnea, cough, fatigue, and loss of appetite (3,24). NSCLC is also associated with higher rates of emotional distress compared with other malignancies (4). These symptoms all can adversely affect quality of life and become progressive during the terminal stages of the disease course. Pain and dyspnea both are common symptoms among patients with advanced NSCLC but can be very responsive to palliative interventions (Figure 1).
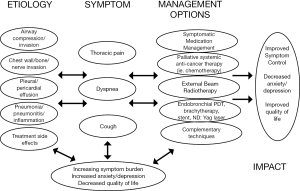
Pain
Pain is experienced by many patients with advanced NSCLC and can cause anxiety, a sense of hopelessness, and depression (25). Pain is often multifactorial and can arise from the tumor itself or from the therapies intended to treat the NSCLC (Table 1). Lung cancer patients experience higher rates of pain than patients with other primary cancer diagnoses (26-28). Among 1,453 cancer patients who completed quality of life questionnaires after hospitalization, patients with lung cancer had the highest mean pain level of the 11 most prevalent cancer diagnoses (26). Furthermore, pain prevalence has been reported in 74-90% of patients with lung cancer (27,29). These higher rates compared with other malignancies may be related to a more frequent use of multiple and concurrent treatment modalities for NSCLC, higher likelihood of metastatic disease spread, and poorer clinical outcome compared with other malignancies (2).
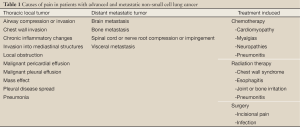
Full Table
In most patients, malignant pain can be well managed with medical interventions that optimize analgesic medications, doses, and dose intervals (30,31). Despite this, many patients with cancer receive inadequate pain management (32-36). Medical professionals treating NSCLC patients with advanced and metastatic disease should make pain management a priority and assess pain symptoms at each patient encounter.
Dyspnea
Patients experience dyspnea as an uncomfortable sensation or awareness of restricted breathing. From 46-90% of patients with NSCLC experience dyspnea (37,38). Dyspnea occurs both from the tumor and therapies used to treat the underlying malignancy (14,24). Palliative management of dyspnea can improve quality of life among NSCLC patients (39). Dyspnea can be relieved via palliative procedures or cancer-directed therapies. Patients with symptomatic tumor invasion or obstruction of the central airway can be considered for endoscopic palliation interventions, including laser therapy, stent placement, and endobronchial brachytherapy (40,41). Symptomatic pleural effusions can be managed with palliative thoracentesis, catheter or chest tube drainage, chemical pleurodesis, or pleurectomy. Medical interventions, such as bronchodilators, opioids, diuretics, supplemental oxygen, training in breathing techniques, pulmonary rehabilitation, and chest wall vibration, can also improve patient-reported dyspnea (42).
Phase III randomized studies of patients with advanced-stage NSCLC have shown that systemic therapy can improve dyspnea, as well as fatigue, cough, and general quality of life (43,44). However, chemotherapy can also result in dyspnea in up to one in five patients treated and should be continuously reevaluated (45). Radiation therapy can also often achieve improvements in dyspnea but can similarly result in treatment-induced dyspnea from radiation pneumonitis or pulmonary fibrosis. The role of palliative radiotherapy in symptom management is discussed in more detail later in this manuscript.
Palliative systemic therapy
In addition to early palliative care initiation improving survival, improvements in chemotherapy over the past 15 years have allowed for prolongation of survival of patients with stage IV NSCLC by several months compared with supportive care alone, older chemotherapeutic regimens, or single-agent therapy (46-48).
Newer third-generation cytotoxic agents such as pemetrexed for adenocarcinomas and gemcitabine for squamous cell carcinomas, as well as vinorelbine, paclitaxel, and docetaxel, have antitumor activity for advanced-stage NSCLC. Among first-line therapy studies, a randomized trial of 432 advanced-stage NSCLC patients demonstrated that the addition of vinorelbine to cisplatin increased the response rate (26% vs. 12%, P=0.0002), median progression-free survival (4 vs. 2 months, P=0.0001), and median overall survival (8 vs. 6 months, P=0.0018) (49). Another randomized trial of 522 advanced-stage NSCLC patients showed that the addition of gemcitabine to cisplatin increased the response rate (30.4% vs. 11.1%, P<0.0001), median progression-free survival (5.6 vs. 3.7 months, P=0.0013), and median overall survival (9.1 vs. 7.6 months, P=0.004) (50).
Based on the improvements in both quality of life and overall survival, current clinical practice guidelines recommend that all patients with advanced or metastatic NSCLC with good performance status be evaluated for systemic therapy (51,52). Platinum-based regimens have been demonstrated in numerous studies to prolong survival compared with best supportive care alone, and cisplatin is preferable to carboplatin in candidates for that agent (53). First-line therapy for advanced or recurrent NSCLC should consist of chemotherapy alone or in combination with bevacizumab in patients with good performance statuses (54), and erlotinib can be administered to patients who have a mutation in the epidermal growth factor receptor. While doublet chemotherapy can improve survival compared with single-agent chemotherapy, three concurrent cytotoxic drugs typically do not improve survival and are associated with increased toxicity. However, combining target agents like bevacizumab or cetuximab with doublet chemotherapy can further improve outcomes. Guidelines also suggest considering maintenance therapy in all patients with stage IV NSCLC, and such maintenance regimens should continue until evidence of disease progression or their use is limited by toxicity.
There is no consensus on the use of cytotoxic chemotherapy among elderly patients or those with limited performance statuses, and their use can be associated with patient morbidity. Stage IV NSCLC patients who are elderly or have limited performance statuses should be considered for single-agent chemotherapy with third-generation drugs such as vinorelbine, gemcitabine, or taxanes, or for targeted therapies (51,55).
There has also been an improvement in second-line agents for the treatment of refractory or progressive NSCLC. Newer agents such as erlotinib, docetaxel, or pemetrexed (particularly for adenocarcinomas) have shown efficacy in this setting and can improve quality of life and prolong overall survival. A meta-analysis of 24 randomized trials found that patients with recurrent or progressive NSCLC achieve improvements in overall survival and quality of life with single-agent docetaxel and single-agent pemetrexed, and with erlotinib for patients not eligible for further chemotherapy (56). Epidermal growth factor tyrosine kinase inhibitors like erlotinib, vascular endothelial growth factor receptor inhibitors like bevacizumab, anaplastic lymphoma kinase inhibitors like crizotinib, and other targeted systemic therapies are increasingly being utilized to treat refractory NSCLC and can be administered as single agents or in combination with standard chemotherapy (57,58).
Palliative external beam radiation therapy
Palliative radiation therapy can be used in an attempt to symptomatically improve localized symptoms from disease. Although radiation therapy is commonly used in patients with stage I-III NSCLC for curative intent, palliative irradiation is not used in an attempt to achieve a cancer cure. As the majority of patients with NSCLC either present with or develop metastasis during the course of their disease (2), palliative irradiation is a commonly used modality. In fact, a recent population-based United States Surveillance, Epidemiology, and End Results study of 11,084 Medicare beneficiaries with metastatic NSCLC found that 58% of patients received palliative radiation therapy (59).
For patients with advanced or metastatic NSCLC, palliative thoracic external beam radiotherapy is well tolerated and can achieve symptomatic response rates of approximately 75-95% for hemoptysis, 60-85% for chest pain, 50-60% for cough, and 40-60% for dyspnea (60). Acute and subacute toxicities such as nausea and emesis, lung hemorrhage, severe esophagitis, Lhermitte’s syndrome, and bronchoesophageal fistulas occur in less than 1% of patients, and radiation pneumonitis and chronic pulmonary fibrosis with respiratory insufficiency are similarly rare in doses typically delivered for palliation (60-63).
When definitive standard-fractionation external beam radiation therapy is used for curative intent for NSCLC, doses of 60 Gy or more are delivered to sites of gross disease and areas at risk of microscopic disease spread. In contrast, palliative external beam radiation therapy to thoracic disease is typically administered only to areas of gross disease to a dose of 20 Gy in five fractions or 30 Gy in 10 fractions. For sites of bone metastasis without spinal cord compression, a single fraction of palliative radiotherapy is often used to allow for improved patient convenience, expedited treatment completion, and a reduction in healthcare costs (64).
In an attempt to define the optimal dose and fractionation regimens for palliative external beam radiation therapy in patients with NSCLC, multiple randomized trials have been performed assessing outcomes, including overall survival and responses of pain, dyspnea, cough, hemoptysis, atelectasis and post-obstructive pneumonia to radiotherapy (65-67). In a review of 14 randomized trials that compared differing regimens of palliative radiation therapy for NSCLC, no single regimen was found to be optimal for symptomatic palliation, and symptomatic response rates did not differ based on the number of fractions administered. Of note, patients with good performance statuses had improved survival rates by 5% at 1 year and 3% at 2 years with higher palliative radiation doses, although such regimens resulted in more acute toxicity, including esophagitis. Patients with poorer performance statuses may be best treated with short radiation therapy courses of one or two fractions (68).
Recent similar recommendations of using shorter courses to provide more rapid and convenient symptoms relief versus more protracted courses that are optimally delivered to patients with good performance statuses to provide more durable symptomatic relief and potentially an improvement in overall survival have been issued by the American College of Radiology Appropriateness Criteria (61) and the American Society for Radiation Oncology (63).
A cost-utility analysis of differing radiation therapy fractionation regimens was assessed in a randomized trial comparing 30 Gy in 10 fractions and 16 Gy in two fractions in 297 patients with advanced or metastatic NSCLC. Patients treated in the more protracted 10-fraction arm had greater quality-adjusted life years (20.0 vs. 13.2 weeks, P=0.05) largely due to an improvement in survival in patients receiving 10 fractions (38.1 vs. 27.4 weeks, P=0.03). Although the radiation therapy costs per patient were more than twice as much for patients treated in 10 fractions, survival-related non-radiotherapy cost offset this difference due to the shorter life expectancy for patients treated in only two fractions, thus allowing for 30 Gy in 10 fractions to be determined to be acceptable according to current economic standards (69).
Palliative endobronchial therapies
Advanced NSCLC with local tumor progression can result in symptomatic central airway obstruction or invasion that can lead to life-threatening respiratory compromise or hemoptysis. Endobronchial treatments can allow for rapid symptomatic relief and are often well tolerated with minimal toxicity. The optimal treatment of central obstruction depends on the type of obstruction. Endoluminal obstruction can be palliated with brachytherapy, laser therapy, or photodynamic therapy, extraluminal obstruction with brachytherapy or stent placement, and infiltrating lesions with brachytherapy, laser therapy, or stenting (70,71). External beam radiation therapy, however, can often achieve symptom relief for any type of central airway obstruction.
Compared with external beam radiation therapy, endobronchial brachytherapy generally allows for higher irradiation doses to be delivered to the tumor with less volume and dose to surrounding normal tissues, potentially allowing for fewer acute toxicities. Endobronchial brachytherapy administered alone or in combination with external beam radiation therapy to 95 patients with locally advanced NSCLC has been reported to improve patient-reported quality of life and functional capacity and achieve symptomatic response rates of 97% for hemoptysis, 93% for dyspnea, 91% for obstructive pneumonia, and 81% for cough (72). Therefore, based on the available literature and risk of severe toxicity, endobronchial brachytherapy may be most ideally suited for patients who are symptomatic from recurrent endobronchial central obstruction and who have previously been treated with external beam radiation therapy.
However, a meta-analysis of 13 randomized trials of central obstructions compared different regimens of palliative external beam radiation therapy, endobronchial brachytherapy, and other endobronchial interventions. The combination of endobronchial brachytherapy with external beam radiation therapy did not improve symptomatic control or overall survival compared with external beam radiation therapy alone (73). Similarly, a meta-analysis of 29 trials of endobronchial brachytherapy in NSCLC found external beam radiation therapy alone to be more effective than brachytherapy for symptom palliation in previously untreated patients (74). External beam radiation therapy may also allow for a more durable palliative response and less need for additional interventions compared with brachytherapy due to decreased tumor progression outside the bronchus (75). Brachytherapy is also less suited to treat large tumors or adjacent lymph node metastasis than external beam radiation therapy, and it may have a higher risk of late toxicities like massive hemoptysis, tracheoesophageal fistula formation, bronchial stenosis, and bronchospasms (76).
Photodynamic therapy is another intervention that can achieve palliative resolution or improvement in dyspnea and pulmonary function related to endobronchial luminal obstruction and tumor-related stenosis. Photodynamic therapy can also resolve acute hemoptysis and poststenotic pneumonia. Photodynamic therapy has minimal toxicities and can be used safely in combination with other palliative techniques, including external beam radiation therapy (77). Among 100 patients with advanced or metastatic lung cancer with endobronchial luminal obstruction treated with photodynamic therapy, the mean endoluminal obstruction decreased from 85.8% to 17.5% after therapy, and patients similarly had significant improvements in mean forced vital capacity and forced expiratory volume in one second (78). In another study of 133 symptomatic patients with endobronchial lung lesions treated with photodynamic therapy, a significant improvement in dyspnea was achieved in 74% of patients as assessed by the Modified Medical Research Council Dyspnea Scale (79).
Nd:YAG laser therapy is the most widely utilized modality for tumor ablation within the tracheobronchial tree. In a study of 258 advanced lung cancer lesions causing endobronchial stenosis or obstruction, patients were treated with Nd:YAG laser therapy (n=177) or photodynamic therapy (n=81). The overall treatment effectiveness was 81% for Nd:YAG laser and 75% for photodynamic therapy, with Nd:YAG laser being more effective for tumors in the trachea or main bronchi (93% vs. 73%) but not for tumors in lobar or segmental bronchi (73% vs. 76%). Nd:YAG therapy, however, did result in massive bleeding in 6%, perforations in 3%, and a procedural mortality rate of 1.7%, compared with no major complications after photodynamic therapy (80). Overall, with limited comparative data available, Nd:YAG laser therapy and photodynamic therapy appear to have similar efficacy in relieving intraluminal tumor obstruction and improving patient symptoms, but photodynamic therapy may allow for a longer time to treatment failure and more limited toxicity profile (77).
Palliation of sites of NSCLC metastasis
Metastases to the brain, adrenal glands, and liver are frequent occurrences in patients with advanced or metastatic NSCLC and often cause symptoms like pain or neurologic, liver, or adrenal dysfunction. Furthermore, in addition to bone metastases that can cause pain and decrease functional mobility, spinal metastases can cause spinal cord or nerve root impingement or compression that can lead to decreased strength, decreased sensation, or loss of bowel or bladder function. Such metastases can decrease overall survival and often carry a high symptom burden that can significantly worsen patient quality of life and symptom scores. Palliation of such metastases can be achieved via medical management and is often directed towards specific symptoms. In addition, patients with symptomatic brain metastasis can be management with systemic steroids, antiepileptics, whole-brain radiation therapy, stereotactic radiosurgery, and neurosurgical resection. Patients with symptomatic adrenal or liver metastasis can be managed with surgery, stereotactic body radiotherapy, palliative external beam radiation therapy, radiofrequency ablation or other ablative techniques, transarterial chemoembolization, and systemic therapy. Patients with spinal cord compression should be treated with systemic steroids and undergo surgical management that is often followed by external beam radiation therapy, or radiotherapy alone in patients with more limited life expectancies or who are not candidates for surgery (14).
Conclusions
NSCLC often presents at an advanced stage and is the leading cause of death attributed to cancer worldwide. Many patients with NSCLC experience symptoms from their cancer or their cancer therapy. Palliative care for NSCLC should be implemented early in the course of the disease and should to be conducted through a multispecialty approach. Early integration of palliative care for patients with advanced for metastatic NSCLC can reduce patient symptoms, improve quality of life, and prolong survival. Engagement with patients and their families around issues including illness understanding, coping, and symptom management appear to be important communication tasks to help patients through the course of their illness.
The addition of systemic therapy, including chemotherapy or targeted therapy, to supportive care improves quality of life and prolongs survival. Systemic therapy should strongly be considered in all patients, particularly those with good performance statuses. Newer first-line and second-line therapies are further prolonging survival among metastatic and refractory or progressive NSCLC patients.
Thoracic external beam radiation therapy is well tolerated and can improve quality of life and relieve symptoms such as hemoptysis, pain, cough, and dyspnea. More protracted external beam radiation therapy regimens may provide more durable symptomatic response and prolong survival, whereas shorter courses provide similar symptomatic response rates and should be considered in patients with poorer performance statuses and more limited life expectancies. For central airway obstruction, external beam radiation therapy, endobronchial brachytherapy, ablation with Nd:YAG laser, and photodynamic therapy can be used, and the choice of therapy should be tailored to the time of obstruction and individual patient. External beam radiation therapy is a preferred first-line option over endobronchial brachytherapy, whereas endobronchial brachytherapy is optimally administered for previously irradiated symptomatic patients with central obstruction. Further study is needed to determine how palliative radiotherapy should be best integrated with systemic therapy. Symptomatic metastasis to the brain, adrenal glands, and liver, and spinal cord or nerve root compression or impingement occur often in patients with NSCLC, and management should be site specific and can include medical and pharmacologic interventions, radiation therapy, surgery, ablative therapy, and chemotherapy.
All healthcare providers treating patients with advanced or metastatic NSCLC should make palliative care a priority by assessing for pain, dyspnea, and other symptoms at each patient encounter to reduce patient morbidity, improve quality of life, and potentially prolong survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- American Joint Committee on Cancer. Lung. AJCC Cancer Staging Manual Seventh Edition. Chicago, IL, USA: Springer, 2010:253-70.
- Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer 1995;71:633-6. [PubMed]
- Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol 2000;18:893-903. [PubMed]
- Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol 2009;27:5816-22. [PubMed]
- Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005;23:6865-72. [PubMed]
- Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009;27:206-13. [PubMed]
- Jordhøy MS, Fayers P, Loge JH, et al. Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol 2001;19:3884-94. [PubMed]
- Morita T, Akechi T, Ikenaga M, et al. Late referrals to specialized palliative care service in Japan. J Clin Oncol 2005;23:2637-44. [PubMed]
- WHO Definition of Palliative Care. Available online: http://www.who.int/cancer/palliative/definition/en/
- Ferris FD, Bruera E, Cherny N, et al. Palliative cancer care a decade later: accomplishments, the need, next steps--from the American Society of Clinical Oncology. J Clin Oncol 2009;27:3052-8. [PubMed]
- Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients’ families. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. JAMA 1994;272:1839-44. [PubMed]
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009;115:2013-21. [PubMed]
- Simone CB II. The role of palliative care in patients with NSCLC. Curr Med Lit Lung Cancer 2011;5:1-16.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- Connor SR, Pyenson B, Fitch K, et al. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage 2007;33:238-46. [PubMed]
- Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. [PubMed]
- Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10:865-71. [PubMed]
- Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. [PubMed]
- The NCCN Clinical Practice Guidelines in Oncology™ Palliative Care (Version 2.2013). National Comprehensive Cancer Network, Inc. Available online: www.NCCN.org
- Clinical practice guidelines for quality palliative care, Third Edition. National Consensus Project for Quality Palliative Care. Available online: http://www.nationalconsensusproject.org/NCP_Clinical_Practice_Guidelines_3rd_Edition.pdf
- Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 2011;14:459-64. [PubMed]
- Yoong J, Park ER, Greer JA, et al. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med 2013;173:283-90. [PubMed]
- Temel JS, Pirl WF, Lynch TJ. Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer 2006;7:241-9. [PubMed]
- Spiegel D, Sands S, Koopman C. Pain and depression in patients with cancer. Cancer 1994;74:2570-8. [PubMed]
- Rustøen T, Fosså SD, Skarstein J, et al. The impact of demographic and disease-specific variables on pain in cancer patients. J Pain Symptom Manage 2003;26:696-704. [PubMed]
- Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer 2004;90:2288-96. [PubMed]
- Greenwald HP, Bonica JJ, Bergner M. The prevalence of pain in four cancers. Cancer 1987;60:2563-9. [PubMed]
- Simone CB 2nd, Vapiwala N, Hampshire MK, et al. Palliative care in the management of lung cancer: analgesic utilization and barriers to optimal pain management. J Opioid Manag 2012;8:9-16. [PubMed]
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65-76. [PubMed]
- Goisis A, Gorini M, Ratti R, et al. Application of a WHO protocol on medical therapy for oncologic pain in an internal medicine hospital. Tumori 1989;75:470-2. [PubMed]
- Simone CB 2nd, Vapiwala N, Hampshire MK, et al. Internet-based survey evaluating use of pain medications and attitudes of radiation oncology patients toward pain intervention. Int J Radiat Oncol Biol Phys 2008;72:127-33. [PubMed]
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994;330:592-6. [PubMed]
- Thomason TE, McCune JS, Bernard SA, et al. Cancer pain survey: patient-centered issues in control. J Pain Symptom Manage 1998;15:275-84. [PubMed]
- Cleeland CS, Janjan NA, Scott CB, et al. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys 2000;47:203-8. [PubMed]
- Simone CB 2nd, Vapiwala N, Hampshire MK, et al. Cancer patient attitudes toward analgesic usage and pain intervention. Clin J Pain 2012;28:157-62. [PubMed]
- Reddy SK, Parsons HA, Elsayem A, et al. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med 2009;12:29-36. [PubMed]
- Mohan A, Singh P, Kumar S, et al. Effect of change in symptoms, respiratory status, nutritional profile and quality of life on response to treatment for advanced non-small cell lung cancer. Asian Pac J Cancer Prev 2008;9:557-62. [PubMed]
- Gupta D, Lis CG, Grutsch JF. The relationship between dyspnea and patient satisfaction with quality of life in advanced cancer. Support Care Cancer 2007;15:533-8. [PubMed]
- Skowronek J, Kubaszewska M, Kanikowski M, et al. HDR endobronchial brachytherapy (HDRBT) in the management of advanced lung cancer--comparison of two different dose schedules. Radiother Oncol 2009;93:436-40. [PubMed]
- Scarda A, Confalonieri M, Baghiris C, et al. Out-patient high-dose-rate endobronchial brachytherapy for palliation of lung cancer: an observational study. Monaldi Arch Chest Dis 2007;67:128-34. [PubMed]
- Bausewein C, Booth S, Gysels M, et al. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 2008;(2):CD005623. [PubMed]
- Grønberg BH, Bremnes RM, Fløtten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217-24. [PubMed]
- Helbekkmo N, Strøm HH, Sundstrøm SH, et al. Chemotherapy and quality of life in NSCLC PS 2 patients. Acta Oncol 2009;48:1019-25. [PubMed]
- West HL, Wakelee HA, Perry MC, et al. Gemcitabine and pemetrexed administered in rapid sequence as front-line chemotherapy for advanced non-small-cell lung cancer: a phase II clinical trial. Ann Oncol 2009;20:850-6. [PubMed]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;(5):CD007309. [PubMed]
- Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res 1998;4:1087-100. [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [PubMed]
- Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 1998;16:2459-65. [PubMed]
- Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2000;18:122-30. [PubMed]
- The NCCN Clinical Practice Guidelines in Oncology™ Non-small Cell Lung Cancer (Version 2.2013). National Comprehensive Cancer Network, Inc. Available online: www.NCCN.org
- de Marinis F, Rossi A, Di Maio M, et al. Treatment of advanced non-small-cell lung cancer: Italian Association of Thoracic Oncology (AIOT) clinical practice guidelines. Lung Cancer 2011;73:1-10. [PubMed]
- Sculier JP, Moro-Sibilot D. First- and second-line therapy for advanced nonsmall cell lung cancer. Eur Respir J 2009;33:915-30. [PubMed]
- Rinaldi M, Cauchi C, Gridelli C. First line chemotherapy in advanced or metastatic NSCLC. Ann Oncol 2006;17 Suppl 5:v64-7. [PubMed]
- Gridelli C, Maione P, Rossi A, et al. Management of unfit older patients with advanced NSCLC. Cancer Treat Rev 2009;35:517-21. [PubMed]
- Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2006;1:1042-58. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Hayman JA, Abrahamse PH, Lakhani I, et al. Use of palliative radiotherapy among patients with metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;69:1001-7. [PubMed]
- Kepka L, Olszyna-Serementa M. Palliative thoracic radiotherapy for lung cancer. Expert Rev Anticancer Ther 2010;10:559-69. [PubMed]
- Rosenzweig KE, Movsas B, Bradley J, et al. ACR appropriateness criteria on nonsurgical treatment for non-small-cell lung cancer: poor performance status or palliative intent. J Am Coll Radiol 2009;6:85-95. [PubMed]
- Reinfuss M, Mucha-Małecka A, Walasek T, et al. Palliative thoracic radiotherapy in non-small cell lung cancer. An analysis of 1250 patients. Palliation of symptoms, tolerance and toxicity. Lung Cancer 2011;71:344-9. [PubMed]
- Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol 2011;1:60-71. [PubMed]
- Maher EJ, Coia L, Duncan G, et al. Treatment strategies in advanced and metastatic cancer: differences in attitude between the USA, Canada and Europe. Int J Radiat Oncol Biol Phys 1992;23:239-44. [PubMed]
- Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70. [PubMed]
- Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167-75. [PubMed]
- Bezjak A, Dixon P, Brundage M, et al. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 2002;54:719-28. [PubMed]
- Lester JF, Macbeth FR, Toy E, et al. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev 2006;(4):CD002143. [PubMed]
- van den Hout WB, Kramer GW, Noordijk EM, et al. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst 2006;98:1786-94. [PubMed]
- Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1-6. [PubMed]
- Stephens KE Jr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg 2000;119:289-96. [PubMed]
- Mallick I, Sharma SC, Behera D. Endobronchial brachytherapy for symptom palliation in non-small cell lung cancer--analysis of symptom response, endoscopic improvement and quality of life. Lung Cancer 2007;55:313-8. [PubMed]
- Cardona AF, Reveiz L, Ospina EG, et al. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2008;(2):CD004284. [PubMed]
- Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 2006;5:189-202. [PubMed]
- Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol 2000;56:323-7. [PubMed]
- Perez CA, Brady LW. eds. Principles and Practice of Radiation Oncology. 2nd Edition. Philadelphia: J.B. Lippincott, 1992:50-63, 114-23.
- Simone CB 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis 2012;4:63-75. [PubMed]
- Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1-6. [PubMed]
- Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg 2010;89:1744-8; discussion 1748-9.
- Furukawa K, Okunaka T, Yamamoto H, et al. Effectiveness of Photodynamic Therapy and Nd-YAG Laser Treatment for Obstructed Tracheobronchial Malignancies. Diagn Ther Endosc 1999;5:161-6. [PubMed]


