Self-expanding metallic stents and self-expanding plastic stents in the palliation of malignant oesophageal dysphagia
Charles stent (1) would have been very proud to know that his last name has been immortalised and still frequently used in the fields of gastrointestinal endoscopy, cardiology, urology and gynaecology. What started as a dental mould then evolved into today’s sophisticated self-expanding stents.
Self-expanding metallic wallstent was first used in 1990 for relieving malignant oesophageal obstruction (2). This was then essentially a compressed woven wire mesh, made of surgical grade stainless steel alloy filaments. However, subsequent reports of tumour ingrowth (3) with such stents led to the production of a silicon membrane covered stent to overcome this problem. The following years saw many more improved modifications to the self-expanding metallic stent (SEMS), in the attempt to achieve an ideal stent. Each one of these stents has its own unique features (see Table 1) to cater to the individual need.
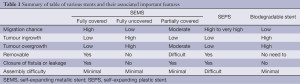
Full table
Self-expanding metallic stent (SEMS) (oesophageal)
In the palliation of malignant dysphagia, SEMS has proven to be superior to the rigid plastic stents with less complications (3% vs. 43%), lower mortality (14% vs. 29%), and better relief of dysphagia (80% vs. 62-69%) (4,5). Except for self-expanding plastic stent (SEPS), SEMS have replaced the rigid plastic stents in the endoscopic management of malignant dysphagia. Endoscopic stenting of oesophageal and upper gastrointestinal malignancies have been proven to be safe, efficacious and cost effective (6,7).
SEMS are available commercially from several companies, each with their own special unique features, to cater to the individual need. The main common features of a SEMS include a woven metallic wire mesh cylinder, compressed within a small diameter delivery system, with/without a silicon/plastic membrane covering the mesh interstices. Most of the oesophageal SEMS are deployed over a guidewire outside the scope, though Taewoong Medical has commercialised an oesophageal SEMS that could be deployed through the scope (similar to that of a duodenal or colonic SEMS). Most SEMS are deployed by manually unsheathing the outer tube containing the inner compressed stent. The Evolution controlled release SEMS (Cook Medical Endoscopy Inc, USA) are released by pulling the trigger of a gun handle; every trigger pull unsheathing a fixed 6 mm length of the stent. However, it remains to be seen whether such design can result in higher technical success rate of oesophageal stenting, as compared to conventional release method.
Verschuur et al. compared three different types of stents, partially covered Ultraflex stent (Boston Scientific, USA), polyflex stent (Boston Scientific, USA) as well as the fully covered Niti-S stents (Taewoong Medical, Seoul, Korea) for the palliation of malignant dysphagia (8). Technical success rate was 100%, 83% and 95% respectively. Dysphagia improved in all patients with no differences in complications. However, recurrent dysphagia (from tissue ingrowth/overgrowth or migration or food obstruction) was 52% vs. 37% vs. 31%. Stent migration was highest in those with polyflex stents whereas tissue ingrowth/overgrowth occurred more in Ultraflex stents and less in Niti-S stents. The conclusion was that polyflex stents are technically more difficult to deploy and with the highest chance of stent migration. We could also conclude that a covered stent is probably associated with less risk of tumour ingrowth/overgrowth.
Uncovered SEMS
This SEMS has no silicon or plastic membrane covering the wire mesh interstices. The underlying oesophageal tissues will, with time, become embedded within the stent mesh. As the result, the migration chance is minimal. The main problem of such stent is the risk of tumour ingrowth due to the exposed wire interstices and it cannot be safely removed after deployment. Subsequent endoscopic intervention, including laser boring of tumour within the stent, or redeployment of a 2nd stent within the original stent, has to be carried out for tumour ingrowth/overgrowth.
Covered SEMS
Due to the presence of silicon membrane covering the wire mesh interstices, this SEMS is associated with a low chance of tumour ingrowth. Vakil et al. reported similar technical success and dysphagia relief but tumour ingrowth of 3% vs. 30% in covered Ultraflex vs. uncovered Ultraflex SEMS (9). Removal of this covered stent was also very easy though CE and FDA approval for benign oesphageal stricture varies with different stents (see Table 2). It is this easy removability that makes this covered SEMS suitable in trachea-oesophageal fistula or leakage. Neoadjuvant radiotherapy planning with CT scan and even oesophagectomy can be hindered with the presence of a stent and hence this removable stent is also suitable for such temporary deployment. However, this feature also results in the high chance of stent migration (7% to 25%) (10,11), with recurrence of dysphagia symptom and the need to retrieve the migrated stent.
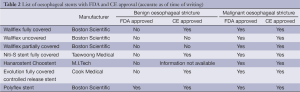
Full table
Partially covered SEMS
This SEMS attempts to reduce the migration risk of deployed stent, and yet reduce the chance of tumour ingrowth (8). The covered portion is usually in the centre, with the uncovered portion at the proximal and distal ends. However, the risk of migration, compared to uncovered stent, and the risk of tumour ingrowth/overgrowth, compared to covered stent are still relatively higher.
Self-expanding plastic stent (SEPS)
This is a device with an encapsulated monofilament braid made of polyester. The meshes are completely covered by a silicone layer with a smooth inner surface and a more structured outer surface. This stent has to be loaded with a large 13 mm diameter introducer sheath before deployment and the assembly is rather cumbersome. The obvious main advantage of this stent is the easy removal and hence it is, like the covered SEMS, suitable in tracheo-oesophageal fistula and in cases where temporary relief of obstruction is indicated. In fact, it has been approved for refractory benign oesophageal stricture with initial good encouraging outcome (12-14) of 90% clinical relief and migration chance of 6.7% to 52.4%. However, a recent retrospective study of 83 deployments in 30 patients with benign stricture/fistula revealed a 62.1% stent migration with 81.9% patients reporting recurrence or persistence of symptoms during long term follow-up (15). It is this high migration risk and cumbersome assembly with a large introducer that makes the SEPS less favoured over covered SEMS in relieving oesophageal obstruction. It is both FDA and CE approved for the treatment of both malignant and benign oesophageal stricture (see Table 2).
A few unique features of certain SEMS deserve to be mentioned here.
Anti-reflux features
Up to one third of patients with distal oesophageal stenting across the GE junction experiences significant acid reflux symptoms (16). The polyurethane coating of some SEMS is extended beyond the distal end to form a windsock valve. The Dua antireflux stent (Cook Medical Endoscopy Inc, USA) in canine model, has been shown to reduce oesophageal acid reflux from 49% to 1% (17). However, a randomised controlled trial comparing this stent with other stents without anti reflux features did not show any significant improvement in reflux symptoms (18-20). These anti-reflux features are also available in Niti-S stent (Taewoong Medical Co, Seoul, Korea) and Hanarostent (M.I.Tech, Seoul, Korea).
Anti-migration features
The double layered Niti-S oesophageal stent (Taewoong Medical, Seoul, Koroea) comprises of a silicon membrane covered inner layer and a wire mesh external layer. The wire mesh will gradually embed within the underlying tissues and hence reduce the chance of migration while the covered inner layer reduces tumour ingrowth and facilitates smooth passage of food through the stent.
M.I.Tech oesophageal SEMS (Choostent and Hanarostent) comprised of segmented stent structure with larger bands than others. This unique feature is supposedly useful to reduce migration chance. Davide Bona et al. (21) compared Choostent vs. covered Ultraflex oesophageal stents and reported a 4.6% migration rate in Choostent. Though there were two Ultraflex migration compared to one Choostent, these were not statistically significant. The Choostents were also reported to be easily removed up to eight week post deployment under sedation.
Use in upper oesophageal obstruction
Stenting of the upper oesophagus continues to be shunned by many endoscopists who are concerned about intolerable throat pain, stridor, aspiration pneumonia and stent migration into the hypopharynx. Small studies have shown that 28% of patients experienced intolerable throat pain when the standard SEMS was placed 1.5 cm from the upper esophageal sphincter (UES), as compared to none when the proximal limit is 2 cm (22,23). A large retrospective study of 104 patients with oesophageal stenting near the UES reported 33% complications including aspiration pneumonia and 15% intolerable throat pain (24).
Most SEMS are released from the distal end to the proximal end. For stenting of the upper oesophagus, accurate placement is crucial due to the small margin of error allowed as stent encroachment onto the upper oesophageal sphincter may lead to stridor or significant throat pain. Boston Scientific has an Ultraflex stent that can be released proximally so that satisfactory position can be determined with confidence before the irreversible full deployment.
A special cervical stent is available (Taewoong Medical and M.I.Tech) comprising a much smaller 7 mm proximal funnel in an obtuse angle. These features appear to relieve the throat discomfort frequently experienced after cervical oesophageal stenting using a conventional stent. From my personal experience, no significant discomfort will be felt as long as the proximal end of the stent is deployed not nearer than 1.5 cm from the upper oesophageal sphincter (25).
Biodegradable stents
Biodegradable oesophageal stents have been recently developed in the aim to obviate the need to remove the stent after deployment for temporary relief of obstruction. The first case series published on the use of biodegradable stents made of poly-L-lactic acid monofilaments (for oesophageal cancers after surgery or endoscopic submucosal dissection) reported stent migration of 77% (10 out of 13) within 10-21 days of deployment (26). However, all the migrated stents were passed out in the stools and no symptoms of re-stenosis were observed in any patients two years later.
The SX-Ella Esophageal Stent Degradable BD (Ella-CS, Czech Republic) is a stent completely made of polydioxanone, a crystalline biodegradable polymer. This material is partly absorbable and partly excreted through the gastrointestinal tract lumen and the stent is supposed to start degrading after 4-5 weeks. Van Boeckel et al. compared this BD stent to Polyflex SEPS and reported similar rates of long term relief of dysphagia but the BD stent was associated with few re-intervention as it need not be removed (27). Repici et al. reported a 9.5% stent migration with all stents completely dissolved after six months deployment (28). However, a few serious complications including tracheo-oesophageal fistula and collapse of stent mesh have been reported (29,30).
Overall, BD stents appear to be a viable alternative to SEPS and SEMS for cases where temporary relief is needed. We await more studies to establish its efficacy and safety.
Conclusions
Self-expanding metallic and plastic stents are now used worldwide and are essential tools in the endoscopy armamentarium. So what is the ideal stent? Perhaps the ideal oesophageal stent for palliation of malignant dysphagia should have the following features:
- No assembly required before deployment;
- Strong radial expansile force for tight stricture;
- Minimal foreshortening;
- Durable material with low risk of stent fracture after deployment;
- Easy to deploy with easily identifiable markers endoscopically or fluoroscopically;
- Able to re-sheath or re-adjust during or immediately after deployment;
- Small diameter of delivery system so that it could be deployed through the standard accessory channel;
- Low risk of stent migration;
- Low risk of tumour ingrowth and overgrowth;
- Low risk of acid reflux if stenting has to traverse the GE junction;
- Ability to close up fistula, leakage effectively;
- Biodegradable negating the need for re-intervention to remove the stent. Better still, able to initiate easy self-degradation with a simple ingestion of a tablet or simple endoscopic flushing with a safe dissolution reagent;
- Low cost to patients.
Many of the current SEMS have many of these ideal properties. I am confident that we may soon see the ideal SEMS available for the management of both benign and malignant oesophageal dysphagia.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Ambekar S, Nanda A. Charles Stent and the mystery behind the word “stent”. J Neurosurg 2013;119:774-7. [PubMed]
- Domschke W, Foerster EC, Matek W, et al. Self-expanding mesh stent for esophageal cancer stenosis. Endoscopy 1990;22:134-6. [PubMed]
- Bethge N, Knyrim K, Wagner HJ, et al. Self-expanding metal stents for palliation of malignant esophageal obstruction--a pilot study of eight patients. Endoscopy 1992;24:411-5. [PubMed]
- Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302-7. [PubMed]
- Sanyika C, Corr P, Haffejee A. Palliative treatment of oesophageal carcinoma--efficacy of plastic versus self-expandable stents. S Afr Med J 1999;89:640-3. [PubMed]
- Yim HB, Jacobson BC, Saltzman JR, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 2001;53:329-32. [PubMed]
- Dormann A, Meisner S, Verin N, et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy 2004;36:543-50. [PubMed]
- Verschuur EM, Repici A, Kuipers EJ, et al. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol 2008;103:304-12. [PubMed]
- Vakil N, Morris AI, Marcon N, et al. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol 2001;96:1791-6. [PubMed]
- Ko HK, Song HY, Shin JH, et al. Fate of migrated esophageal and gastroduodenal stents: experience in 70 patients. J Vasc Interv Radiol 2007;18:725-32. [PubMed]
- Binmoeller KF, Maeda M, Lieberman D, et al. Silicone-covered expandable metallic stents in the esophagus: an experimental study. Endoscopy 1992;24:416-20. [PubMed]
- Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg 2005;79:398-403; discussion 404. [PubMed]
- Repici A, Conio M, De Angelis C, et al. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc 2004;60:513-9. [PubMed]
- Radecke K, Gerken G, Treichel U. Impact of a self-expanding, plastic esophageal stent on various esophageal stenoses, fistulas, and leakages: a single-center experience in 39 patients. Gastrointest Endosc 2005;61:812-8. [PubMed]
- Holm AN, de la Mora Levy JG, Gostout CJ, et al. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest Endosc 2008;67:20-5. [PubMed]
- Valbuena J. Endoscopic palliative treatment of esophageal and cardial cancer: a new antireflux prosthesis. A study of 40 cases. Cancer 1984;53:993-8. [PubMed]
- Dua KS, Kozarek R, Kim J, et al. Self-expanding metal esophageal stent with anti-reflux mechanism. Gastrointest Endosc 2001;53:603-13. [PubMed]
- Homs MY, Wahab PJ, Kuipers EJ, et al. Esophageal stents with antireflux valve for tumors of the distal esophagus and gastric cardia: a randomized trial. Gastrointest Endosc 2004;60:695-702. [PubMed]
- Wenger U, Johnsson E, Arnelo U, et al. An antireflux stent versus conventional stents for palliation of distal esophageal or cardia cancer: a randomized clinical study. Surg Endosc 2006;20:1675-80. [PubMed]
- Blomberg J, Wenger U, Lagergren J, et al. Antireflux stent versus conventional stent in the palliation of distal esophageal cancer. A randomized, multicenter clinical trial. Scand J Gastroenterol 2010;45:208-16. [PubMed]
- Bona D, Laface L, Bonavina L, et al. Covered nitinol stents for the treatment of esophageal strictures and leaks. World J Gastroenterol 2010;16:2260-4. [PubMed]
- Macdonald S, Edwards RD, Moss JG. Patient tolerance of cervical esophageal metallic stents. J Vasc Interv Radiol 2000;11:891-8. [PubMed]
- Shin JH, Kim SW, Shim TS, et al. Malignant tracheobronchial strictures: palliation with covered retrievable expandable nitinol stent. J Vasc Interv Radiol 2003;14:1525-34. [PubMed]
- Verschuur EM, Kuipers EJ, Siersema PD. Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest Endosc 2007;66:1082-90. [PubMed]
- Yim HB. Stenting of malignant upper oesophageal obstruction: experience in a regional hospital in Singapore. Eur J Gastroenterol Hepatol 2011;23:948-51. [PubMed]
- Saito Y, Tanaka T, Andoh A, et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenterol 2007;13:3977-80. [PubMed]
- van Boeckel PG, Vleggaar FP, Siersema PD. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol 2011;9:653-9. [PubMed]
- Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc 2010;72:927-34. [PubMed]
- Jung GE, Sauer P, Schaible A. Tracheoesophageal fistula following implantation of a biodegradable stent for a refractory benign esophageal stricture. Endoscopy 2010;42 Suppl 2:E338-9. [PubMed]
- Nogales Rincon O, Huerta Madrigal A, Merino Rodriguez B, et al. Esophageal obstruction due to a collapsed biodegradable esophageal stent. Endoscopy 2011;43 Suppl 2 UCTN:E189-90.


