Self-expandable metallic stent placement for palliation in gastric outlet obstruction
Introduction
Even today, many gastrointestinal (GI) malignancies are significantly advanced and incurable at presentation. Unresectable malignancies frequently lead to luminal obstruction, and reobstruction due to local recurrence or lymph node metastasis may occur after surgical resection. Gastric outlet obstruction (GOO) particularly occurs in patients with unresectable peri-ampullary (e.g., pancreatic, ampullary, hepatobiliary cancer) or gastric cancer.
The consequences of GOO can be serious. These include intolerance of oral intake and deterioration of quality of life (QOL), with vomiting, aspiration, bloating and malnutrition. Surgical gastrojejunostomy (GJJ) has been performed as a conventional palliative procedure for GOO, but the disadvantages of this procedure include significant risks of higher morbidity and mortality (1), and a higher incidence of delayed gastric emptying (2). Enteral stenting has been increasingly used as an alternative to surgical palliation thanks to its lower invasiveness and quicker response, and the many articles related to enteral stenting for GOO show a variety of evidence. This review paper overviews the literature on enteral stenting for GOO.
General outline of gastric outlet obstruction (GOO)
GOO is usually found as a late complication and causes a variety of obstructive symptoms, including nausea, vomiting, or bloating, and usually leads to poor or no oral intake in affected patients. These symptoms tend to lead to dehydration, malnutrition and weight loss, and these are distinguished from cancerous cachexia, which accompanies advanced malignancy. Severe GOO which prevents the passage of gastric juice is often accompanied by electrolyte dehydration as well as dehydration and reflux esophagitis. These symptoms are likely to markedly harm the QOL of affected patients. The goal of palliation of GOO is to resume oral intake and improve obstructive symptoms.
Treatments for malignant GOO
The conventional palliative management for GOO is GJJ, either open or laparoscopic. This procedure provides an effective reduction in obstructive symptoms and allows the resumption of oral intake. However, enteral stent placement was developed in the early 1990’s (3-6) and has been practically available for 15 years now.
In addition to stent placement and bypass surgery, other palliative procedures include chemotherapy, radiotherapy, insertion of a decompression tube (e.g., nasogastric or gastrostomy tube), and administration of somatostatin analogue. These have been used independently or in combination with stent placement or GJJ. Nevertheless, the only effective management which allows the resumption of oral intake is surgical GJJ and stent placement; in the absence of either, patients are usually unable to ingest food orally, and often require placement of a decompression tube.
Surgical palliation carries significant risks of morbidity and mortality (1), and frequently causes delayed gastric emptying (2). In addition, many patients with GOO are poor surgical candidates, because of their debilitated condition and malnutrition due to significantly advanced cancer. Against this background, stent placement is both effective in palliating GOO and minimally invasive, and is now widely used in these patients.
Types of enteral stents
Enteral stents used for GOO consist of a metal alloy (e.g., nitinol) mesh in a cylindrical shape, and are termed self-expandable metallic stent (SEMS). Most SEMS used in the gastroduodenal region have a knitted or braided wire structure. Several types of SEMS which differ with regard to mesh structure and properties (radial force, axial force, etc.) are now commercially available from various manufacturers. SEMSs can be flared at the proximal or both ends, and may be covered with a polyurethane or polytetrafluoroethylene membrane to help prevent tumor ingrowth.
For insertion, the stent is constrained and loaded into the delivery system, most of which are designed for through-the-scope (TTS) deployment. This delivery system is about 10-Fr, which allows passage through the working channel of therapeutic endoscopes. However, SEMSs with a larger introducer sheath designed for over-the-wire (OTW) deployment are also available in some countries (7). OTW deployment is usually performed by radiologists.
Placement procedure
Before the development of dedicated devices, anatomical difficulties made stent placement for GOOs a difficult and challenging procedure (3-6). The development of dedicated stents and TTS placement have markedly facilitated placement, however, even in long, tortuous strictures.
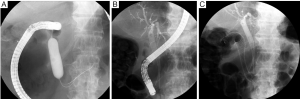
Currently, stent placement is mostly performed with the TTS deployment technique because of its significant ease of use (8) (Figure 1). In addition, TTS deployment technique has an advantage enabling simultaneous placement of two stents without second insertion of endoscope (Figure 2). However, the diameter of the delivery catheter is 10-10.5 Fr, requiring a therapeutic endoscope with a large working channel. The procedure is performed under conscious sedation and analgesia. The prone position is optimal because it avoids aspiration and allows an ideal X-ray image to be taken. The X-ray tube of the C-arm should be appropriately rotated so that side view of the stenosis can be obtained. A therapeutic endoscope with a large working channel is inserted and the stenosis is observed. It is not necessary to traverse the stenosis with the endoscope if the stenosis is tight. Negotiation of the stricture is performed using a biliary guidewire (usually “0.035” in diameter) with an ERCP catheter. Once the guidewire can be passed through the stricture, sufficient contrast is injected to define the length of the stenosis. Withdrawing the catheter/guidewire from the distal to the proximal end of the stenosis, or use of a measuring guidewire, is helpful in determining the precise length. An appropriate length of stent (usually at least 2 cm longer than the measured stricture at each end) is then chosen according to the length of the stenosis to prevent tumor overgrowth. The stent delivery system is inserted along the guidewire through the working channel of the endoscope. The stent is deployed at the stenotic region in consideration of the foreshortening ratio of the stent, which varies with stent type. The stent should be gradually deployed, with adjustment for position. After deployment, proper positioning is confirmed by a waist within the SEMS. Further, passage is determined by contrast injection via the endoscope. An abdominal plain X-ray film is taken daily to confirm stent positioning and the degree of expansion. Full expansion is usually obtained within three days.
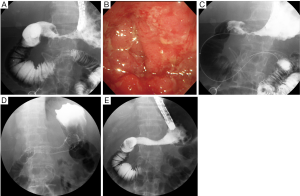
Indications and contraindications
Placement of an enteral stent is indicated in patients with documented malignant obstruction of the pylorus and/or duodenum caused by unresectable tumors. Stent placement is frequently employed in patients who are poor surgical candidates with shortened life expectancy, poor performance status, significant comorbidities and anesthetic risk (9,10).
Contraindications of this procedure are evidence of GI perforation and documentation of multiple distal obstructions, particularly in the small bowel. Peritoneal carcinomatosis may induce multiple distal obstructions, but a study found that a diagnosis of carcinomatosis only should not be considered a contraindication to SEMS placement in patients with malignant GOO (11).
Efficacy
This procedure with TTS deployment is not difficult, and has a technical success rate of 90% to 100% (12-20). A review of 1,046 published cases reported a technical success rate of 96% (21). The most common causes of technical failure were unsuccessful transit of the guidewire through the stenosis, failed placement of the SEMS at the proper position, and migration of the SEMS during the procedure.
Clinical success, defined as the relief of obstructive symptoms and improvement in oral intake, is obtained in 58% to 92% of patients (12-20). The above review article indicates a clinical success rate of 89% (21). The discrepancies between technical success and clinical success might be attributable to underlying GI dysmotility with or without neural involvement by the tumor, distal obstruction secondary to peritoneal carcinomatosis, or general deconditioning and anorexia caused by advanced malignancy (9). A study which assessed whether stent location alters efficacy revealed that efficacy was not altered by location of the stent across the pyloric valve or within the duodenum (22).
Oral intake is most frequently assessed using the Gastric Outlet Obstruction Scoring System (GOOSS), with 0= no oral intake, 1= liquid only, 2= soft solids, and 3= low-residue or full diet (23). Many articles suggested that GOOSS score is significantly improved following stent placement (14,15,18,19,21,24-27). Most patients can continue oral intake until death. A recent study revealed that 95.9% of patients continued oral intake for the rest of their lives and that 78.4% required no further intervention until death (24). This study also revealed that many patients can resume solid food intake (GOOSS 2 or 3), with a cumulative average of 74%, ranging from 56% to 80% (15,16,24,27,28). In addition, approximately two-thirds of patients continued solid food intake until death (24). A study evaluating predictive factors of solid food intake showed that a Karnofsky performance score of 50% or less and the presence of ascites are independent poor predictive factors of ability to ingest solid food (29).
According to a functional evaluation study (30), almost 80% of patients studied had a significant improvement in gastric emptying rate. Nevertheless, another study using radionuclide scanning indicated that gastric emptying function in patients one week after stenting was significantly poorer than in healthy subjects (31).
Quality of life (QOL)
A prospective randomized trial comparing duodenal stenting versus laparoscopic GJJ by Mehta and colleagues (32) showed a significant improvement in physical health score at one month (P<0.01), but no change in pain score or mental health score at this time. No improvement in any QOL parameter was seen in the laparoscopic GJJ group. Another comparative study conducted under a retrospective design indicated that an improvement in Karnofsky performance score was more frequent in the stent group than in GJJ group (65% vs. 26.3%, P=0.0248) (33). Further, the median difference in performance score before and after the procedure was significantly greater in the stent group than in the bypass group (15 vs. –10; P=0.0149) (33). A UK study by Lowe and colleagues reported similar results, with an increase in Karnofsky score from 44/100 to 63/100 post-procedure (34). A prospective study with the WallFlex stent by van Hooft and colleagues indicated a significant improvement in post-procedural WHO performance score between the pre-stenting score and mean score up to death (14).
A study which objectively evaluated QOL score before and after stenting using the EORTC QLQ-C30 instrument to assess functional status and cancer-related symptoms and the QLQ-STO22 instrument to assess gastric-specific symptoms found that among QLQ-C30 parameters, role functioning, physical functioning, global health status, and nausea/vomiting improved after stenting, although the difference was statistically significant only for global health status (P=0.010) and nausea/vomiting (P=0.001). In contrast, however, no change was seen in other QLQ-C30 parameters, including emotional, cognitive, and social functions, or other symptoms (35). In addition, enteral stenting was associated with a significant improvement in dysphagia (P=0.001), eating restrictions (P=0.010), dry mouth (P=0.029), and reflux (P=0.040), as assessed by the QLQ-STO22 instrument (35).
One group has recently reported three prospective studies of three different SEMSs, namely the DUOFLEX (WallFlex stent) (14), DUONITI (Niti-S stent) (18) and DUOLUTION (Evolution stent) (25) studies. The QOL score results of the three studies differed, but it is unclear whether this was due to the different structures of the stents (Table 1).
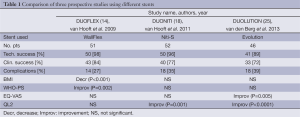
Full table
Complications and management
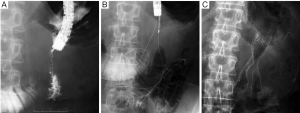
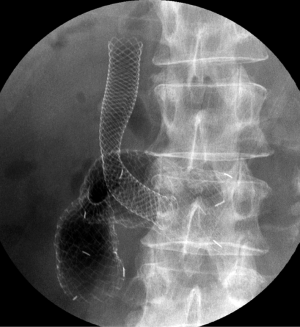
Complications are frequently classified as either early- (≤7 days) or late-stage changes (>7 days). According to a systematic review (21), major early complications, including migration and stent dysfunction, occur in 7%, and major late complications in 18%. The most common causes are stent migration, and obstruction caused by tumor in- or over-growth, hyperplasia, or food impaction. Obstruction (5-21.1%) is more frequent than migration (0-3.8%) (14-16,18,19,34). Tumor-related stent obstructions can be managed by placement of a second stent (Figure 3) or ablative procedures (36), while migration is often treated by placement of an additional stent. Minor complications, such as pain, nausea or vomiting, are not frequent (9%) (21), while life-threatening complications like perforation and bleeding are rare (1% or less) (9,37). SEMS with significant flexibility and blunt ends may be helpful in preventing ulcer formation and perforation (13).
Combination with biliary stent placement
Biliary obstruction can occur concurrently with GOO, or before or after GOO. Both gastroduodenal and biliary obstructions are classified into three patterns based on timing and location (Table 2). Mutignani and colleagues proposed a classification for the duodenal stenosis type in relation to the major papilla, with type I at a level proximal to and without involvement of the papilla; type II affecting the second part of the duodenum with involvement of the papilla; and type III involving the third part of the duodenum distal to and without involvement of the papilla (38).

Full table
Biliary obstruction usually occurs in patients with pancreaticobiliary malignancy as the underlying disease, but sometimes also in patients with other etiologies, such as gastric, duodenal or metastatic cancers. Particularly in patients with pancreaticobiliary malignancies, biliary obstruction tends to develop before the occurrence of GOO. One study reported the onset of biliary obstruction before GOO in 56%, concomitantly in 25%, and following the development of GOO in 19% (10,23). Many patients undergoing enteral stenting for GOO thus already have a pre-existing biliary stent to manage a preceding biliary obstruction. In these cases, if the pre-existing biliary stent is a plastic, it should be replaced with a SEMS, given the risk of buckling and inability to retrieve it. In type II patients with preceding biliary SEMS, concern has been expressed about the possible blockage of bile outflow with the use of a covered duodenal SEMS. A study which compared post-procedural bilirubin and alkaline between covered and uncovered SEMSs placed to bridge the papilla concluded that placement of a covered SEMS was not contraindicated (39). Nevertheless, selection of an uncovered SEMS to avoid the endoscopic inaccessibility of the bile duct may be preferable.
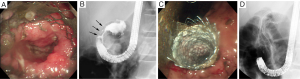
In cases in which biliary obstruction is concomitant with GOO, simultaneous placement of a biliary stent should be considered when placing an enteral stent for GOO, since the success rate of this procedure is comparable to that of placement of a duodenal stent alone (40). In cases with either simultaneous or two-stage placement, biliary stenting prior to duodenal stenting should be considered (Figure 4), because endoscopic biliary stenting is generally impossible when a duodenal stent bridges the papilla. If transpapillary biliary stenting fails even with the use of balloon dilation for duodenal stricture, a percutaneous or EUS-guided transmural approach (41) may be selected (Figure 5).
As stated above, development of a biliary obstruction after a duodenal obstruction is least common. Thanks to the pre-existing enteral stent, the duodenoscope can usually reach the level of the major papilla. In cases with an enteral SEMS bridging the papilla, however, a transpapillary approach is often impossible.
Stent placement versus gastrojejunostomy (GJJ)
Many studies, including three randomized studies, have compared enteral stenting and GJJ (32,33,42-55). Most have suggested the superiority of enteral stenting, particularly with regard to short-term outcomes such as a shorter hospital stay and faster resumption of oral intake. The most recent systematic review reported similar results (56). Another systematic review, however, found that although stenting had a higher clinical success rate and fewer minor complications, it had a higher rate of recurrence of obstructive symptoms, suggesting that stenting may be more favorable in patients with a relatively short life expectancy, while GJJ is preferable in those with a longer prognosis (21). These authors also conducted the largest randomized study to date (53), the results of which were consistent with their previous systematic review (21). This study showed that enteral stenting was associated with poorer long-term results, with more major complications (6 vs. 0 cases; P=0.02) and a higher incidence of recurrent obstructive symptoms (8 vs. 1; P=0.02) and reinterventions (10 vs. 2; P<0.01), versus a better short-term outcome, with more rapid improvement of oral intake (5 vs. 8 days; P<0.01) and a shorter hospital stay (7 vs. 15 days; P=0.04) (53). There was no difference in median survival or QOL scores (53). The authors again proposed that enteral stenting should be considered in patients with a short life expectancy (less than two months). In their subsequent study evaluating possible predictors of survival, WHO score was the only significant predictor of survival in patients with malignant GOO (57). They proposed that patients with WHO score of 0-1 should be considered for GJJ, whereas those with a WHO score of 3-4 should be considered for enteral stenting (57). Similar results were reported in a recent study comparing outcomes between enteral stenting and GJJ only in patients with gastric cancer but a good performance status. That study concluded that enteral stenting was associated with more frequent late adverse events (44.4% vs. 12.2%; P<0.001) and reinterventions (43% vs. 5.5%; P <0.001), and shorter patency (125 vs. 282 days; P=0.001) and survival (189 vs. 293 days; P=0.003) (55), suggesting that enteral stenting is likely favorable in patients with a poor performance status and/or short life expectancy. However, patients with malignant GOO have a limited median survival time (49-99 days) even in many recent literatures (12,14-16,18,19,25,28,58), so many patients have a very short life span and are better served by stents.
The two modalities are compared in Table 3.

Full table
Role of chemotherapy
Some reports have shown that chemotherapy is associated with a lower risk of reobstruction and more frequent migration (12,59). However, a retrospective study comparing clinical outcomes by stent type and chemotherapy for GOO due to gastric cancer revealed that patency rates are significantly improved by combining the use of an uncovered stent with follow-up chemotherapy treatment, because chemotherapy significantly lowered re-intervention rates, particularly with uncovered stents (60). According to a recent study investigating the association between the response to chemotherapy and pyloric stent outcome in patients with gastric cancer, a long time-to-progression (adjusted hazard ratio, 0.29; 95% CI, 0.13-0.67) and first-line chemotherapy (adjusted hazard ratio, 0.45; 95% CI, 0.22-0.93) were significant protective factors against reobstruction, whereas response to chemotherapy was not associated with stent migration or reobstruction (61).
Comparison between stents
Few reports have compared stent outcomes between stent types. In a retrospective study comparing Niti-S with Ultraflex, the former SEMS could be placed by a simpler and faster method, but was more frequently reobstructed (62). Although many enteral stents with different structures are now commercially available, the association between the mechanical properties of stent design and clinical outcome is still poorly understood.
Aside from stent structure or properties, several types of covered SEMS have been developed to reduce the potential risk of stent obstruction due to tumor ingrowth or mucosal hyperplasia. Five studies have compared covered or uncovered SEMS (58,63-66) (RCT, 2; prospective cohort, 1; retrospective cohort, 2). Two Korean studies showed similar results, namely less frequent reobstruction and more frequent migration for covered stents (63,65). However, a retrospective study of covered and uncovered Ultraflex stents showed that covered SEMS were associated with a higher reintervention rate despite similar outcomes in reobstruction and migration (64). A retrospective study with various covered or uncovered SEMSs in patients with pancreaticobiliary malignancies concluded that the use of uncovered SEMS may be preferable for duodenal obstruction secondary to pancreaticobiliary malignancy, since these were effective in preventing stent migration and tended to have a longer patency than covered stents (66). The most recent prospective randomized trial reported that use of a triple-layered covered SEMS was associated with less frequent stent dysfunction at more than four weeks after stenting, despite similar short-term outcomes (58). These conflicting results may be due to differences in patient demographics, stent types, or patient survival period. In any case, they mean that a consensus on the benefit of covered SEMS has yet to be obtained. A larger randomized study is warranted.
Summary
GOO can dramatically detract from QOL. Enteral stenting is beneficial in obtaining a rapid improvement in obstructive symptoms and can be performed with a high success rate. However, it carries a higher risk of late-developing complications than surgical palliation and is therefore likely more favorable in patients with a short life expectancy. Follow-up chemotherapy may significantly lower reintervention rates, particularly with uncovered SEMSs. A consensus regarding the most suitable stent type for GOO and the significance of the use of covered SEMS has yet to be obtained.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Weaver DW, Wiencek RG, Bouwman DL, et al. Gastrojejunostomy: is it helpful for patients with pancreatic cancer? Surgery 1987;102:608-13. [PubMed]
- Doberneck RC, Berndt GA. Delayed gastric emptying after palliative gastrojejunostomy for carcinoma of the pancreas. Arch Surg 1987;122:827-9. [PubMed]
- Kozarek RA, Ball TJ, Patterson DJ. Metallic self-expanding stent application in the upper gastrointestinal tract: caveats and concerns. Gastrointest Endosc 1992;38:1-6. [PubMed]
- Topazian M, Ring E, Grendell J. Palliation of obstructing gastric cancer with steel mesh, self-expanding endoprostheses. Gastrointest Endosc 1992;38:58-60. [PubMed]
- Truong S, Bohndorf V, Geller H, et al. Self-expanding metal stents for palliation of malignant gastric outlet obstruction. Endoscopy 1992;24:433-5. [PubMed]
- Maetani I, Ogawa S, Hoshi H, et al. Self-expanding metal stents for palliative treatment of malignant biliary and duodenal stenoses. Endoscopy 1994;26:701-4. [PubMed]
- Song HY, Shin JH, Yoon CJ, et al. A dual expandable nitinol stent: experience in 102 patients with malignant gastroduodenal strictures. J Vasc Interv Radiol 2004;15:1443-9. [PubMed]
- Soetikno RM, Lichtenstein DR, Vandervoort J, et al. Palliation of malignant gastric outlet obstruction using an endoscopically placed Wallstent. Gastrointest Endosc 1998;47:267-70. [PubMed]
- ASGE Standards of Practice Committee, Fukami N, Anderson MA, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011;74:13-21. [PubMed]
- Brimhall B, Adler DG. Enteral stents for malignant gastric outlet obstruction. Gastrointest Endosc Clin N Am 2011;21:389-403. [PubMed]
- Mendelsohn RB, Gerdes H, Markowitz AJ, et al. Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction. Gastrointest Endosc 2011;73:1135-40. [PubMed]
- Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc 2007;66:256-64. [PubMed]
- Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc 2007;66:355-60. [PubMed]
- van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc 2009;69:1059-66. [PubMed]
- Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol 2009;104:2404-11. [PubMed]
- Shaw JM, Bornman PC, Krige JE, et al. Self-expanding metal stents as an alternative to surgical bypass for malignant gastric outlet obstruction. Br J Surg 2010;97:872-6. [PubMed]
- Kim YW, Choi CW, Kang DH, et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci 2011;56:2030-6. [PubMed]
- van Hooft JE, van Montfoort ML, Jeurnink SM, et al. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy 2011;43:671-5. [PubMed]
- Costamagna G, Tringali A, Spicak J, et al. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis 2012;44:37-43. [PubMed]
- Havemann MC, Adamsen S, Wøjdemann M. Malignant gastric outlet obstruction managed by endoscopic stenting: a prospective single-centre study. Scand J Gastroenterol 2009;44:248-51. [PubMed]
- Jeurnink SM, van Eijck CH, Steyerberg EW, et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 2007;7:18. [PubMed]
- Cheng HT, Lee CS, Lin CH, et al. Treatment of malignant gastric outlet obstruction with metallic stents: assessment of whether gastrointestinal position alters efficacy. J Investig Med 2012;60:1027-32. [PubMed]
- Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol 2002;97:72-8. [PubMed]
- Canena JM, Lagos AC, Marques IN, et al. Oral intake throughout the patients’ lives after palliative metallic stent placement for malignant gastroduodenal obstruction: a retrospective multicentre study. Eur J Gastroenterol Hepatol 2012;24:747-55. [PubMed]
- van den Berg MW, Haijtink S, Fockens P, et al. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy 2013;45:174-81. [PubMed]
- Fiori E, Lamazza A, Demasi E, et al. Endoscopic stenting for gastric outlet obstruction in patients with unresectable antro pyloric cancer. Systematic review of the literature and final results of a prospective study. The point of view of a surgical group. Am J Surg 2013;206:210-7. [PubMed]
- Isayama H, Sasaki T, Nakai Y, et al. Management of malignant gastric outlet obstruction with a modified triple-layer covered metal stent. Gastrointest Endosc 2012;75:757-63. [PubMed]
- Graber I, Dumas R, Filoche B, et al. The efficacy and safety of duodenal stenting: a prospective multicenter study. Endoscopy 2007;39:784-7. [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Predictive factors of solid food intake in patients with malignant gastric outlet obstruction receiving self-expandable metallic stents for palliation. Dig Endosc 2012;24:226-30. [PubMed]
- Larssen L, Hauge T, Medhus AW. Stent treatment of malignant gastric outlet obstruction: the effect on rate of gastric emptying, symptoms, and survival. Surg Endosc 2012;26:2955-60. [PubMed]
- Maetani I, Ukita T, Tada T, et al. Gastric emptying in patients with palliative stenting for malignant gastric outlet obstruction. Hepatogastroenterology 2008;55:298-302. [PubMed]
- Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc 2006;20:239-42. [PubMed]
- Maetani I, Tada T, Ukita T, et al. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy 2004;36:73-8. [PubMed]
- Lowe AS, Beckett CG, Jowett S, et al. Self-expandable metal stent placement for the palliation of malignant gastroduodenal obstruction: experience in a large, single, UK centre. Clin Radiol 2007;62:738-44. [PubMed]
- Schmidt C, Gerdes H, Hawkins W, et al. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg 2009;198:92-9. [PubMed]
- Maetani I, Tada T, Shimura J, et al. Technical modifications and strategies for stenting gastric outlet strictures using esophageal endoprostheses. Endoscopy 2002;34:402-6. [PubMed]
- Laasch HU, Martin DF, Maetani I. Enteral stents in the gastric outlet and duodenum. Endoscopy 2005;37:74-81. [PubMed]
- Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy 2007;39:440-7. [PubMed]
- Kim SY, Song HY, Kim JH, et al. Bridging across the ampulla of Vater with covered self-expanding metallic stents: is it contraindicated when treating malignant gastroduodenal obstruction? J Vasc Interv Radiol 2008;19:1607-13. [PubMed]
- Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc 2003;17:457-61. [PubMed]
- Khashab MA, Fujii LL, Baron TH, et al. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos). Gastrointest Endosc 2012;76:209-13. [PubMed]
- Yim HB, Jacobson BC, Saltzman JR, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 2001;53:329-32. [PubMed]
- Wong YT, Brams DM, Munson L, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc 2002;16:310-2. [PubMed]
- Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg 2004;28:812-7. [PubMed]
- Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res 2004;24:269-71. [PubMed]
- Mittal A, Windsor J, Woodfield J, et al. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg 2004;91:205-9. [PubMed]
- Maetani I, Akatsuka S, Ikeda M, et al. Self-expandable metallic stent placement for palliation in gastric outlet obstructions caused by gastric cancer: a comparison with surgical gastrojejunostomy. J Gastroenterol 2005;40:932-7. [PubMed]
- Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc 2006;20:1083-7. [PubMed]
- El-Shabrawi A, Cerwenka H, Bacher H, et al. Treatment of malignant gastric outlet obstruction: endoscopic implantation of self-expanding metal stents versus gastric bypass surgery. Eur Surg. 2006;38:451-5.
- Jeurnink SM, Steyerberg EW. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol 2007;96:389-96. [PubMed]
- Chandrasegaram MD, Eslick GD, Mansfield CO, et al. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc 2012;26:323-9. [PubMed]
- Roy A, Kim M, Christein J, et al. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012;26:3114-9. [PubMed]
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 2010;71:490-9. [PubMed]
- Khashab M, Alawad AS, Shin EJ, et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc 2013;27:2068-75. [PubMed]
- No JH, Kim SW, Lim CH, et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc 2013;78:55-62. [PubMed]
- Ly J, O’Grady G, Mittal A, et al. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010;24:290-7. [PubMed]
- Jeurnink SM, Steyerberg EW, Vleggaar FP, et al. Predictors of survival in patients with malignant gastric outlet obstruction: a patient-oriented decision approach for palliative treatment. Dig Liver Dis 2011;43:548-52. [PubMed]
- Maetani I, Mizumoto Y, Shigoka H, et al. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc 2014;26:192-9. [PubMed]
- Cho YK, Kim SW, Hur WH, et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci 2010;55:668-74. [PubMed]
- Park CI, Kim JH, Lee YC, et al. What is the ideal stent as initial intervention for malignant gastric outlet obstruction? Dig Liver Dis 2013;45:33-7. [PubMed]
- Kim CG, Park SR, Choi IJ, et al. Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction. Endoscopy 2012;44:807-12. [PubMed]
- Maetani I, Ukita T, Nambu T, et al. Comparison of ultraflex and niti-s stents for palliation of unresectable malignant gastroduodenal obstruction. Dig Endosc 2010;22:83-9. [PubMed]
- Lee KM, Choi SJ, Shin SJ, et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol 2009;44:846-52. [PubMed]
- Maetani I, Ukita T, Tada T, et al. Metallic stents for gastric outlet obstruction: reintervention rate is lower with uncovered versus covered stents, despite similar outcomes. Gastrointest Endosc 2009;69:806-12. [PubMed]
- Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc 2010;72:25-32. [PubMed]
- Woo SM, Kim DH, Lee WJ, et al. Comparison of uncovered and covered stents for the treatment of malignant duodenal obstruction caused by pancreaticobiliary cancer. Surg Endosc 2013;27:2031-9. [PubMed]


