Thoracic reirradiation for lung cancer: a literature review and practical guide
Introduction
Lung cancer (LC) remains the most common cancer, with approximately 1.8 million cases diagnosed in 2012 worldwide (1). Due to earlier detection, advances in staging, increasing use of combined modality treatment, and advances in radiation therapy (RT), patients with LC are also living longer (2). Unfortunately, approximately 50% will experience locoregional, marginal and/or distant failure post-treatment (3,4). After curative-intent chemoradiotherapy, the five year rate of locoregional recurrence in non-small cell lung cancer (NSCLC) is 30% (5), and in patients with small cell lung cancer (SCLC), local failure occurs in approximately one-third (6). Recurrent LC often causes significant symptom burden, negatively affecting quality of life and representing the most common cause of death (7-13).
Historically, thoracic reirradiation (ReRT) has been limited by toxicity concerns, the possibility of tumour radioresistance, and lack of robust evidence (14). Consequently, ReRT of recurrent LC in past was primarily palliative, implemented to relieve symptoms and/or delay consequences of tumour growth (11), when no other treatment options were available. However, a small subset of patients presenting with localized recurrence are increasingly being offered ReRT for salvage, albeit on an ad hoc basis and largely at the discretion of the treating radiation oncologist.
Our objective was to systematically review the literature regarding thoracic ReRT for LC in order to develop practical guidelines for both palliative- and radical-intent ReRT.
Methods
Publications related to repeat fractionated external beam irradiation (ReRT) of the thorax for primary LC were obtained via literature search (Appendix 1). Medline and Embase were searched for English language articles published in full between 2000 and 2013. Eligible studies and review articles were also identified from reference lists of retrieved papers. Additional clinical practice guidelines and consensus documents were obtained from searching the online SAGE (Standards and Guidelines Evidence) Directory compiled by the Canadian Partnership Against Cancer (www.cancerview.ca).

Full table
Studies investigating fractionated ReRT (EBRT) before or after stereotactic body radiosurgery or which included accelerated EBRT were excluded. Abstracts and studies for which outcomes could not be separated by tumour histology or RT modality were excluded, as were case reports and in silico (treatment planning studies) of single patients. Additionally, one publication (15) described what appeared to be earlier results of the same cohort as an included study (9); determining patient duplication was limited by the data presented, so the earlier paper was excluded.
Spearman’s correlation coefficient explored relationships between RT doses and overall survival (OS).
Results
General
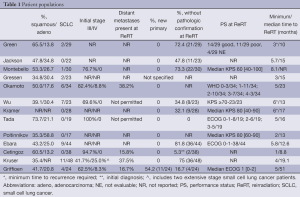
Eleven single-centre retrospective reviews (7,9,12-14,16-21), one prospective cohort study (10) and one phase I/II clinical trial (11) included a total of 379 patients treated with two courses of thoracic EBRT between 1963 and 2013 (Table 1). Excluding the clinical trial, fewer than two (12,13,18,19) to a maximum of eight patients per year (16) received ReRT; Wu et al. recruited 23 patients over two years.
Full table
ReRT was generally defined as a second course of EBRT to an initially radiated volume (9,17), with three reports specifying a minimum interval of three (13) or six (10,11) months after completion of initial treatment. Griffioen required a maximum interval between courses of five years, and two studies stated that the initial EBRT must have delivered a high dose (19) or dose of ≥50 Gy (14). The cohorts of Gressen and Griffioen included new primary lesions (Table 1), which was defined by the latter as either a lesion in a new location, a new histology, or recurrence >5 years after initial treatment. Gressen additionally included patients receiving ReRT for lung metastases although did not specify from which primary tumours (19). Kruser et al. included 8/48 patients whose initial and repeat RT volumes did not overlap in which the second RT course targeted hilar +/- mediastinal lymph node stations not encompassed in upfront treatment. Remaining studies did not define ReRT or locoregional recurrence.
Main demographic and disease factors along with minimum and median time to ReRT are shown in Table 1. Median age ranged from 57 to 71 years, and between 41.2% and 96.4% were male. Between 37.9% and 83.3% of patients had central disease at initial diagnosis (N=4 studies) and just over half had a central recurrence (13). Eight of thirteen studies included SCLC, with only one paper providing separate outcomes (16) (Table 1).
Initial RT
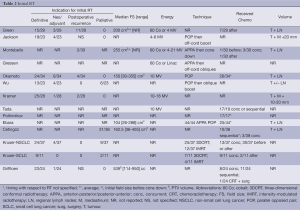
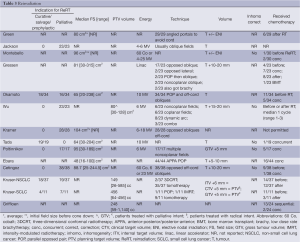
Indications for initial RT included definitive therapy (N=8 studies), neoadjuvant/adjuvant (N=5), to salvage a postoperative recurrence (N=4), or palliative (N=4) (Table 2). From studies reporting intent, greater than half of each cohort was treated with radical, and approximately ¼ with palliative intent, with the exception of one in which 81.6% were treated palliatively (9). EBRT techniques, energies, field sizes (FS), doses and volumes varied significantly as can be expected from these differing treatment indications (Tables 2-4). Some patients were simulated two-dimensionally (2D) under flurosocopy [12/23 (21); 44/44 (17)]. Generally, volumes included the tumour and adjacent mediastinum/regional LN, with some series reporting elective nodal irradiation (ENI). For all cohorts published before 2008, initial RT was delivered 2D, consisting of an anterior-posterior/posterior-anterior (APPA) parallel opposed pair (POP), followed by an off-spinal cord boost with custom blocking. The use of 3D conformal RT (66.7%) and intensity-modulated RT (IMRT) (33.3%) was reported in only one study (16). Half of the Griffioen cohort had their initial RT at another centre, of which 8/12 plans were not available for review (7). A varying number of patients received chemotherapy [sequential (3.3-45.8%), concurrent (7.9-81.8%), or timing not specified] (Table 2). Reported median dose, fraction number and spinal cord doses are shown in Table 4. Biologically equivalent dose was calculated from available reported data.
Full table
Full table
Full table
Reporting of outcomes was limited. Between 6.7% and 21.1% had a complete response (CR) and 40-63.2% a partial response (PR) to initial RT in two studies (18,20). Of 25 patients symptomatic at the time of initial RT, 22 had improvement of at least one symptom, with 100% of those with hemoptysis, superior vena cava obstruction (SVCO), hoarseness and dysphagia responding in one study (20). Toxicity included rates of 12-53% with esophagitis, 47% dry desquamation, up to 32% with pneumonitis and 7% moist desquamation (12,14,20). Authors disagreed on whether patients had to have had a favourable (12,13) response to initial RT to be considered for ReRT, or at least the absence of progression (21); two series retreated patients who did not have a radiographic response after upfront treatment, or progressed during initial RT (18,20).
Patient status prior to ReRT
Various restaging studies prior to ReRT were required by four studies (7,9,11,18). Stringent eligibility criteria in the clinical trial required: minimum performance status (PS), FEV1 >1 L, no severe cardiovascular disease, and normal major organ function (11). Restaging was not required in Green or Kramer and was not specified by remaining. Neither Wu nor Tada permitted distant metastases, and only Tada required pathologic confirmation of disease. In patients without pathologic confirmation, eligibility in the Wu study required recurrence to be confirmed by a group of physicians, while radiographic findings in the Kruser study had to be diagnosed as recurrence by interpreting radiologists. For the remaining, pathologic confirmation was desirable but not essential.
Reirradiation (ReRT)
Indications for repeat irradiation can be divided into four categories: emergent symptomatic, such as SVCO; symptomatic but not emergent, such as dyspnea; asymptomatic but impending serious event, such as airway obstruction; or asymptomatic but with radiological disease progression. Specific indications for ReRT were decided on a case-by-case basis at the discretion of the attending radiation oncologist in each retrospective study. At retreatment, the proportion of symptomatic patients ranged from 42.1% to 100%. In Jackson, Kramer, and Cetingoz, at least 95% of patients were symptomatic, while in Poltinnikov, the figure was just over three-quarters; all patients in these four studies were treated with palliative intent (Table 4).
In the majority of series, ReRT included gross tumour plus a margin (7,9,11,12,14,17-19,21). Significant efforts were made to increase precision of treatment and exclude critical normal organs with maneuvers such as 4D CT simulation (7,16), localized portals (10,13,18), absence of ENI (11,16), immobilization devices (14), consensus gross tumour volume (GTV) determination (11), oblique field arrangements (9,17), use of lesion motion to determine margins (9,11,16), and blocking/multileaf collimation (9,17).
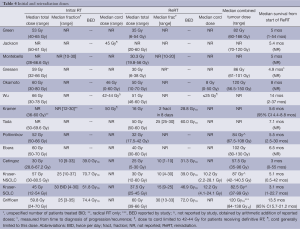
The selection of ReRT dose was at the discretion of the attending physician. It was limited to that sufficient to relieve symptoms in many series (12,17,20), but it was not clear how this was determined. Prescribed doses appeared to be influenced by ReRT intent, initial dose, normal tissue dose and expected lifespan (11,19). For example, Kruser delivered a higher median dose when treating with radical intent (56 Gy/25), compared to those with asymptomatic radiographic progression (20-40 Gy), and those being treated with palliative intent (12-40 Gy). In the phase I/II clinical trial, if the intial RT dose had been <50 Gy, 60 Gy was given at ReRT, and if >50 Gy upfront, 46-50 Gy was delivered (11). Kramer chose a high dose per fraction schedule (16 Gy/2/8 days) to minimize the number of fractions, while Tada used a small dose per fraction to minimize late toxicity. In addition, a handful of patients were reirradiated twice (ie three courses of EBRT total), with cumulative (arithmetic) dose up to 180 Gy.
Ideally, the ReRT planning target volume (PTV) should be covered with the 95% isodose with none of the PTV receiving more than 107% of prescribed dose (11,22). While doses to organs at risk (OARs) were limited to “within tolerance” (not further specified) (4,11), no specific dose limitations were described for the volume of lung, esophagus or heart, other than to spare these as much as possible (10,12). Wu et al. avoided the same beam pathway as the initial RT field arrangement for this reason. High spinal cord doses were accepted by two series if: the patient’s life expectancy was limited; and a high tumour dose was required to control symptoms or prevent a catastrophic outcome such as spinal cord compression (12,21). Spinal cord dose contributed by ReRT was limited to <25-50 Gy by three series (Table 4), although Wu et al. admitted that the constraint lacked firm evidence.
Composite plans were constructed (7,16) when possible and efforts were made to retrieve the original plans for patients treated at an outside institution (Kruser: 14/48). Cumulative dose was estimated with the aid of rigid +/– deformable coregistration (7). Median PTV overlap was 34% (range, 0-96%; N=16) in one study (7). The median degree of overlap of the 50% isodose volumes was 62% (range, 0-95%), and that of the 90% isodose volumes was 44% (range, 0-100%; N=22); however, these dosimetric parameters did not correlate with outcomes (7). Cumulative spinal cord doses were reported by two studies, with Jackson et al. reporting between 30 and 79 Gy received, and Okamoto et al. reporting a median combined dose of 52 Gy and maximum dose of 103 Gy. Most authors mathematically added initial and repeat doses, despite changing dose-fractionation schedules (Table 4). The exceptions to this were Kruser and Griffioen, who calculated equivalent doses in 2 Gy fractions using α/β =10. In the patients conventionally simulated at the time of the second course (9,17,21), little information is available on ReRT or cumulative tumour or OAR doses and composite plans could not be constructed.
Systemic therapy
The rationale for delivery of chemotherapy before, after or concurrent with ReRT was not stated but presumably was at the discretion of the treating oncology team. It is not clear whether the patients who received chemotherapy at the time of ReRT were also those treated with radical intent ReRT, as would be expected. In the prospective Kramer study, all patients were treated with palliative intent and chemotherapy was not permitted, whereas in the Poltinnikov series, all patients were treated with palliative intent, and almost one-third received concurrent chemotherapy. One of thirty-eight patients treated palliatively by Cetingoz also received concurrent chemotherapy and explanation was not provided. In the clinical trial, patients in good condition received one cycle prior to ReRT, although an eligibility criterion was a minimum PS of 70 for all patients (11). Additional sequential cycles were given subsequently if the patient could tolerate. No paper reported use of targeted therapy.
Follow-up
Median follow-up from completion of ReRT ranged from 3.2 to 19.3 months (7,12,17,19,21) although this was variously measured from the start or the completion of repeat RT, or not specified. The majority were followed at the cancer centre until death (7,13,14,21); intensity of follow-up investigations varied, even for patients treated with palliative intent. Most underwent examination every one to three months (11,12,21) by a radiation oncologist and/or respiratory physician, together with CT scan every 3-6 months (12). CT scans were performed 4-6 weeks after the completion of ReRT and then every 8 weeks in another series in which all patients were palliatively treated (14). All patients were seen at 6 and 12 weeks and then every 3 months with exam and CXR after palliative ReRT (10). CT chest was required at 3, 6, and 12 months after ReRT, and after that either CT chest or chest X-ray in the clinical trial (11).
Outcomes: radiologic response
Of patients evaluable for radiographic response, 0-11% had a CR and 7-44% a PR (13,14,20). Of 18 patients given radical-intent ReRT, 6 (33%) had a CR and 8 (44%) had a PR (12). In another study, 5/6 patients receiving chemotherapy after ReRT responded, but neither histology nor FS correlated with radiologic response (13).
Outcomes: symptom response
Symptom improvement rates ranged from just under half (48.3%) to 100% of those assessable, with an overall average of 69.2% either improved or resolved (Table 5). Data on response rates after initial palliative RT and second line systemic therapy are included in the table for comparison. Median duration of symptom response, reported by three series, ranged from 1.8 to 4 months for NSCLC +/– SCLC, and was 0.5 months (range, 0-1.4 months) for SCLC analyzed separately (N=1). As a result of symptom improvement in assessable patients in one study, PS of 9/20 improved and 8/20 stabilized (10). Specific symptom improvement rates were not reported by Wu, Okamoto, Poltinnikov, Kruser, or Griffioen, despite the palliative intent of treatment in most (16), if not all (11,14) of their patients. In at least one series, rates were considered low because of concomitant development of acute pulmonary toxicity and/or symptomatic progressive disease (16). The following did not appear to significantly impact the likelihood of symptomatic benefit from ReRT: histology (21), dose (12,21), specific symptom (12), tumour size (9), or previous RT aim (9).
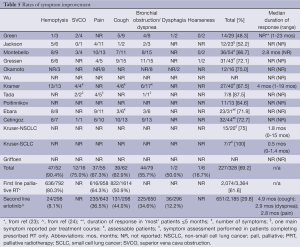
Full table
Outcomes: overall survival (OS)
The majority of patients died of lung cancer (LC) (9,10,12,17,19). Median survival (MS), usually measured from the start of repeat RT, is shown in Table 4. Reported MS in series in which all patients were treated palliatively was approximately 5 months (9,10,14,21). In one study, MS after radical versus palliative-intent ReRT was 15 months (range, 3-58 months) versus 3 months (range, 1-14 months) respectively (12). One year OS ranged from 9-59% (7,9,11,12,16-18) for the NSCLC +/– SCLC populations and 18% for SCLC analyzed separately (16). Median initial, ReRT and combined dose significantly correlated with OS, with Spearman’s rho values of 0.85 (P=0.002), 0.72 (P=0.006), and 0.88 (P=0.001) respectively (Figure 1). Relationships between BED and MS could not be investigated due to insufficient data.

The following factors did not appear to significantly influence OS: improvement in dyspnea (19), age (7,9,19), new primary vs. recurrence (7), initial stage ≥ III vs. < III (7), use of chemotherapy at the time of ReRT in a mixed NSCLC/SCLC population (7), comorbidities (7), degree of initial and repeat PTV overlap (7), dosimetric overlap (7), tumour size <6 or >6 cm (9), or tumour location (central vs. peripheral) (9).
Two studies reported that interval from completion of initial RT to recurrence did not significantly influence OS (7,19), but two studies did (Table 6). Griffioen et al. did not find that PS influenced OS, but three studies did. Griffioen et al. reported that dose did not influence OS but one study did (Table 6).
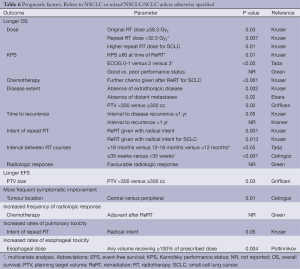
Full table
Outcomes: progression-free survival
One year event-free survival (EFS) was 37% (7) and one year locoregional progression-free survival was 51% (11). Median relapse-free interval after ReRT ranged from 5 to 8.4 months (7,13). In Griffieon, the following did not appear to significantly influence EFS: PS, new primary vs. recurrence, stage ≥III vs. <III, ReRT alone versus chemoRT, age, comorbidities, degree of PTV overlap between initial and ReRT, dosimetric overlap, dose, and interval between RT courses (7).
Outcomes: toxicity
RT-related toxicity and adverse outcomes are seen in Table 7. RT pneumonitis was more frequent after ReRT than after initial EBRT in one study, while the frequency and severity of other RT side effects were similar to those after initial RT (12). In addition to the data in the Table, 6/23 had pulmonary fibrosis on CT chest, of whom four had no symptoms and two were symptomatic (grade three) in one series (11). In another, 6/24 had grade I/II fatigue and 10/24 grade I/II cough (7). In the Kruser series, 2/48 developed bronchostenosis requiring surgery. No publication reported fistula solely as a complication of repeat EBRT.
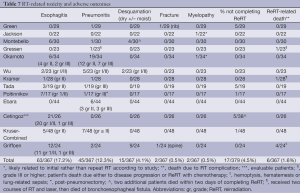
Full table
The following did not appear to influence the rate of pulmonary toxicity: cumulative dose (16), previous or repeat RT dose (17), previous or repeat RT field size (17), interval between courses (17), PS (17), age (17), cumulative FS (17), concurrent chemotherapy (17), or tumour location (17). The length of esophagus within the treatment portal did not influence the likelihood of esophagitis (14). In one series, the single patient who had RT pneumonitis after initial RT did not have a recurrence after repeat RT, and the one patient who developed this after ReRT did not after initial treatment (20).
Due to the development of new symptoms, general deterioration in clinical or PS or death, approximately 5% of patients could not complete the prescribed ReRT (13,14,16,18) (Table 7). In Tada, 4/5 of these patients had PS 3. In Cetingoz, two of the patients abandoned treatment, both dying within the next two months (9). Generally, no descriptors of these patients were reported to help guide patient selection.
One treatment-related death was due to bronchoesophageal fistula following laser treatment two months after ReRT (10), although another patient who underwent laser 8 months after ReRT did not develop fistula. Five over thirteen patients retreated primarily for bleeding died 3-11 months after ReRT from fatal hemoptysis (10). However, because FS were comparable to the rest of the population and none of the patients without hemoptysis at the time of ReRT died of hemoptysis, the authors considered these events tumour-and not treatment-related (10). In Griffioen, three patients were scored as having possible grade 5 toxicity due to either hematemesis or hemoptysis (7). They did not score a fourth fatal event as RT-related (lung-related sepsis after irradiation of a pre-existing cavity); however, without RT it is much less likely that this would have been fatal, so this has been included as a treatment-related death (Table 7). Significant prognostic factors for clinical outcomes are summarized in Table 6.
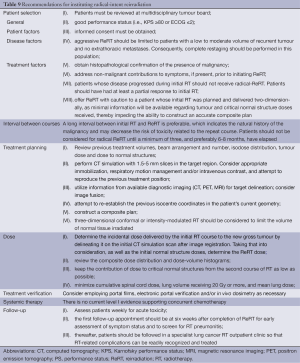
Recommendations
Consideration of many factors is required when determining whether repeat palliative or radical thoracic RT is warranted (Tables 8,9). In both settings, the following should be reviewed, on a case-by-case basis, by a multidisciplinary tumor board: comorbidities, PS, extent of locoregional recurrence, current symptomatology, distant metastases, expected survival, degree of benefit from the first course, likely incremental benefit from and ability to tolerate a second course. Recurrent SCLC should be considered separately, as it is typically aggressive and systemic with a dismal prognosis, especially when symptomatic (16). Tissue sampling to confirm disease recurrence would rule out a non-malignant cause and is highly recommended when considering curative-intent ReRT. Histological diagnosis is also important as the implications, potential treatment options and prognosis for a new primary are significantly different.
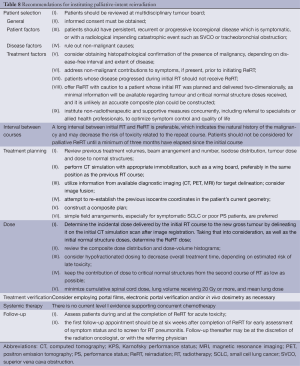
Full table
Full table
Patient and disease characteristics will determine the intent of ReRT, which should be clear to clinician, patient and family. For example, implementation of salvage ReRT should be limited to those who are minimally symptomatic or asymptomatic, with limited local tumour volume, no uncontrolled extrathoracic disease and good PS (20). For palliative ReRT, especially if there are no other treatment options, a potentially effective modality such as ReRT should not be withheld (19). A clear and thorough discussion of the pros and cons should take place and informed consent obtained.
With respect to treatment planning, ReRT intent will determine the reasonable complexity of the plan. Highly conformal EBRT would allow for maximum normal tissue sparing and is advised for radical-intent ReRT. For both radical- and palliative-intent, treatment planning should entail every effort to review previous RT volumes, beam arrangement and number, isodose distribution, and tumor dose. Doses already received by critical normal structures such as the spinal cord and estimation of risk of significant side effects such as RT myelitis will determine the ReRT dose prescription. Composite isodose distribution and dose-volume histograms should be reviewed with attention directed towards minimizing cumulative spinal cord dose, volume of combined normal lung receiving 20 Gy or more, and mean lung dose. For those patients radiated initially with two-dimensional treatment plans, caution is advised due to lack of information about previous doses received.
Conclusive recommendations for follow-up after ReRT have not been established. Many patients treated with palliative intent do not have routine post-RT imaging performed (4). In those who do, distinguishing fibrosis from atelectasis, pneumonic infiltrate, or progression can be difficult (19,20). Follow-up post radical-intent ReRT should adhere to current applicable guidelines for newly diagnosed patients regarding toxicity assessment and options for systemic therapy.
Discussion
To date, the use of ReRT, especially radical-intent, has been controversial. The complexity of implementing ReRT is primarily related to the possibility of causing radiation injury; choosing an appropriate dose in the context of the initial dose and field arrangement; limiting further dose to normal structures which have already received maximum or near-tolerance doses; lack of availability of specific guidelines and data proving efficacy; and a dearth of radiation oncologist experience, with few patients treated per year per institution. The evidence-based recommendations in this report can inform treatment of locoregional recurrence in the palliative and salvage settings, acknowledging the significant heterogeneity in the patient populations, RT, and follow-up practices, and the inherent limitations of retrospective data. Previously published LC treatment guidelines typically recommend RT at the time of recurrence for those who have not previously received it, with skant guidance for ReRT.
The limitations of the data reviewed should be acknowledged. Patients included were highly selected; only 1.5-8.1% of all patients receiving one course of RT were considered eligible for a second course (10,12,13,19,21). Only 11% of patients reviewed had SCLC, and not all series distinguished outcomes separately. The studies describe patients treated over three decades, with outcomes confounded by evolution in diagnosis and imaging.
In the majority of studies published to date, patients were retreated on an ad hoc basis at the discretion of their radiation oncologist without a reported rationale for the choice of dose fractionation, minimum interval between courses, allowed cumulative doses to critical normal structures, or use of chemotherapy. Most studies report the arithmetic cumulative dose (rather than biologically equivalent dose), which does not take into account differences in dose-fractionation or overall treatment time between courses. Treatment techniques, FS, dose calculation procedures and prescription points either vary considerably or are not specified. Complicating the interpretation of results in the palliative ReRT setting is the relatively high dose applied for symptom control (>40 Gy) in some papers and the treatment of asymptomatic patients.
Symptomatic and radiologic response rates may be underrepresented due to the lack of specific definitions and the absence of prospective assessment (10,19,21). There is limited information available about the important parameter of duration of symptom response in relation to survival. In addition, two studies with symptom palliation as the intent of ReRT did not actually report this endpoint (7,11), and response rates of specific symptoms were not provided in others (12,14). It is often difficult to differentiate between symptoms caused by persistent or progressive disease versus those caused by RT-induced parenchymal damage, such as cough and dyspnea (15,25). The rates of specific toxicity in relation to ReRT dose are unclear in the setting of the unknown contribution of the first course to parenchmyal fibrosis. Finally, the often short MS following retreatment does not allow sufficient time for the full extent of late toxicity to manifest (12,20,21); therefore, the degree of risk for the few long-term survivors is unknown.
However, on balance, ReRT should be considered for thoracic recurrence that is currently or imminently symptomatic, if it can be delivered without unacceptable side effects, particularly for those who are expected to achieve a favourable reponse based on results of the previous course (11,13). Radiation oncologists must critically evaluate whether patients are likely to survive long enough to be at risk of late toxicity. A straightforward and thorough discussion must then take place with patients about their options, including an honest appraisal of the potential benefits and potential risks. Only once fully informed patient consent has been obtained should ReRT be delivered.
Recent advances in RT delivery capabilities reduce the volume of normal tissue within treatment fields without compromising tumour coverage. Higher doses may be required to overcome radio-resistance possibly secondary to the presence of chronically hypoxic cells (14,18). Application of 3D conformal or IMRT is essential since these higher initial, repeat and combined doses appear to correlate with improved survival (Figure 1). Since most trials did not analyze outcomes by dose or calculate BED, derivation of robust normal tissue tolerances and correlation of dosimetric parameters with acute and late side effects is not yet possible.
At the time of locoregional recurrence, other treatment options may include stereotactic RT, proton therapy, brachytherapy, boron neutron capture therapy, laser ablation, high linear energy transfer RT, hyperthermia, photodynamic therapy, image-guided ablation, re-resection or systemic therapy (26-35). However, these options are not universally available; each has different and often stringent eligibility criteria; strength of supporting evidence varies; and in some, proof of long-term efficacy is lacking. Table 5 includes comparative data on second line targeted therapy, but further comparison of these modalities is beyond the scope of this review. Finally, the option of best supportive care should not be overlooked.
Our recommendations must be interpreted in the context of the following additional limitations: the type of data available; the likely presence of selection and referral bias; small patient numbers and in some, high attrition rates; short and varying follow-up which affects the incidence of late toxicity; outcomes of patients with SCLC not reported separately in most studies; lack of available quality of life outcomes; and heterogenous baseline characteristics (7,8,14,16). Many studies did not include all radiation details, with the lack of information often due to treatment planning software system changes and evolution of RT delivery techniques (8,17) such as the change from fluoroscopy (2D) to CT-based 3D simulation (16). BED was not reported and data sufficient to calculate it could not be found in most studies, so conclusions regarding dose are limited and confounded by different dose per fraction and overall treatment time.
Conclusions
Despite heterogeneity of patient cohorts, RT techniques and duration of follow-up, ReRT appears to be a feasible option for recurrent thoracic disease, with a small proportion of patients cured by radical-intent ReRT. Treatment guidelines described should guide clinical decision-making. These represent the best evidence-based recommendations which can be derived until further prospective data on modern delivery and planning techniques, and further response, symptom improvement and quality of life data, are available.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Globocan.iarc.fr (homepage on the Internet). International Agency for Research on Cancer, World Health Organization. Fact Sheets by Cancer (updated 2012; cited March 2014). Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2013. Ann Oncol 2013;24:792-800. [PubMed]
- Perez CA, Stanley K, Rubin P, et al. Patterns of tumour recurrence after definitive irradiation for inoperable non-oat cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1980;6:987-94. [PubMed]
- Jereczek-Fossa BA, Kowalczyk A, D’Onofrio A, et al. Three-dimensional conformal or stereotactic reirradiation of recurrent, metastatic or new primary tumours. Strahlenther Onkol 2008;184:36-40. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [PubMed]
- Griffioen GH, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors Lung Cancer 2014;83:356-62. [PubMed]
- Jeremić B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small cell lung cancer: a review. Int J Radiat Oncol Biol Phys 2011;80:969-77. [PubMed]
- Cetingoz R, Arican-Alicikus Z, Nur-Demiral A, et al. Is re-irradiation effective in symptomatic local recurrence of non-small cell lung cancer patients? A single institution experience and review of the literature. J BUON 2009;14:33-40. [PubMed]
- Kramer GW, Gans S, Ullmann E, et al. Hypofractionated external beam radiotherapy as retreatment for symptomatic non-small cell lung carcinoma: an effective treatment? Int J Radiat Oncol Biol Phys 2004;58:1388-93. [PubMed]
- Wu KL, Jiang GL, Qian H, et al. Three-dimensional confirmal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys 2003;57:1345-50. [PubMed]
- Okamoto Y, Murakami M, Yoden E, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys 2002;52:390-6. [PubMed]
- Green N, Melbye R. Lung cancer: retreatment of local recurrence after definitive irradiation. Cancer 1982;49:865-8. [PubMed]
- Poltinnikov IM, Fallon K, Xiao Y, et al. Combination of longitudinal and circumferential three-dimensional esophageal dose distribution predicts acute esophagitis in hypofractionated reirradiation of patients with non-small cell lung cancer treated in stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;62:652-8. [PubMed]
- Çetingöz R, Kentli S, Ataman Ö, et al. Is hypofractionated reirradiation effective after symptomatic local recurrence in non-small cell lung cancer? J BUON 2000;5:421-5.
- Kruser TJ, McCabe BP, Mehta MP, et al. Reirradiation for locoregionally recurrent lung cancer: outcomes in small cell and non-small cell lung carcinoma. Am J Clin Oncol 2014;37:70-6. [PubMed]
- Ebara T, Tanio N, Etoh T, et al. Palliative re-irradiation for in-field recurrence after definitive radiotherapy in patients with primary lung cancer. Anticancer Res 2007;27:531-4. [PubMed]
- Tada T, Fukuda H, Matsui K, et al. Non-small cell lung cancer: reirradiation for locoregional relapse previously treated with radiation therapy. Int J Clin Oncol 2005;10:247-50. [PubMed]
- Gressen EL, Werner-Wasik M, Cohn J, et al. Thoracic reirradiation for symptomatic relief after prior radiotherapeutic management for lung cancer. Am J Clin Oncol 2000;23:160-3. [PubMed]
- Montebello JF, Aron BS, Manatunga AK, et al. The reirradiation of recurrent bronchogenic carcinoma with external beam irradiation. Am J Clin Oncol 1993;16:482-8. [PubMed]
- Jackson MA, Ball DL. Palliative retreatment of locally recurrent lung cancer after radical radiotherapy. Med J Aust 1987;147:391-4. [PubMed]
- Beavis AW, Abdel-hamid A, Upadhyay S. Retreatment of a lung tumour using a simple intensity-modulated radiotherapy approach. Br J Radiol 2005;78:358-61. [PubMed]
- Fairchild A. Chapter 5: side effects of palliative radiation therapy. In: Lutz S, Chow E, Hoskin P. eds. Radiation Oncology in Palliative Cancer Care. Wiley-Blackwell, 2013:43-60.
- Bezjak A, Tu D, Seymour L, Clark G, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group BR21. J Clin Oncol 2006;24:3831-7. [PubMed]
- Fairchild A. Chapter 5: side effects of palliative radiation therapy. In: Lutz S, Chow E, Hoskin P. eds. Radiation Oncology in Palliative Cancer Care. John Wiley & Sons Ltd., 2013:43-60.
- Berman AT, Martin CA, Lin H, et al. Multi-institutional study of reirradiation with proton beam radiotherapy for non-small cell lung cancer. J Clin Oncol 2013;31:abstr 7578.
- Meijneke TR, Petit SF, Wentzler D, et al. Reirradiation and stereotactic radiotherapy for tumours in the lung: dose summation and toxicity. Radiother Oncol 2013;107:423-7. [PubMed]
- Reyngold M, Wu A, McLane A, et al. Toxicity and outcomes of thoracic reirradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol 2013;8:99. [PubMed]
- Ohguri T, Imada H, Yahara K, et al. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: a potential modality for inducing long-term survival in selected patients. Lung Cancer 2012;77:140-5. [PubMed]
- Suzuki M, Suzuki O, Sakurai Y, et al. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy. Int Canc Conf J 2012;1:235-8.
- Trakul N, Harris J, Le Q, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol 2012;7:1462-5. [PubMed]
- Konski A, Chen G, Joiner M, et al. High linear energy transfer (LET) radiotherapy in the treatment of recurrent previously irradiated non-small cell lung cancer. J Thorac Oncol 2011;6:S826-7. Abstract at 14th World Conference on Lung Cancer.
- Leung VA, DiPetrillo TA, Dupuy DE. Image-guided tumour ablation for the treatment of recurrent non-small cell lung cancer within the radiation field. Eur J Radiol 2011;80:e491-9. [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [PubMed]
- Seung S, Solhjem M. Salvage SBRT for previously irradiated lung cancer. Int J Radiat Oncol Biol Phys 2010;78:S537.


