Radiotherapy and radioembolization for liver metastases
Introduction
Liver involvement is common in the setting of metastatic cancer, with disease frequently originating from gastrointestinal (GI) sites and spreading via portal venous drainage, but also commonly from lung, breast, and other primary locations. Liver metastases can, depending on location and size, cause significant pain or obstructive symptoms requiring palliative intervention (1). Beyond strict palliation, select patients, namely those with colorectal primary tumors can benefit from aggressive liver-directed therapy with 5-year survival reported at 30-55% in surgical series (2-5).
While resection has historically served as the primary treatment modality for patients with metastatic or primary liver tumors, many patients are not optimal surgical candidates due to uncontrolled extra-hepatic disease, insufficient (volume of normal) liver tissue, anatomic location of the tumor, or significant medical comorbidities. Additionally, the risk to benefit ratio must be critically evaluated when considering an invasive procedure for a patient with uncertain or poor prognosis. Patients for whom surgical resection is not recommended, or where the intent is palliative, can be offered a variety of locoregional therapies that can achieve local control and improve symptoms. More recently, non-surgical therapies have reported very promising local control rates and even improved survival for select patient subsets (6,7). The spectrum of local therapies includes resection, intratumoral instillation of alcohol or acetic acid, radiofrequency, laser, or cryo-ablation, chemoembolization, conventional radiotherapy (RT), stereotactic body radiotherapy (SBRT), and radioembolization (RE).
In the past, the ability of RT to confer durable tumor response for liver metastases was limited by a relative lack of precision and the possibility of significant toxicity (8). While traditionally delivered RT (usually treating the whole liver without 3-dimensional (3D) treatment planning) could temporarily palliate symptoms, it was historically reserved for symptomatic patients who had failed other therapies (1,9-12). RT has always been the least-invasive local treatment option though doses required to achieve adequate local tumor control could not be safely administered to the entire liver (13) with the risk of radiation induced liver disease (RILD) approaching 50% at a relatively modest dose of 36 Gray (Gy) in 2 Gy fractions (14). Vast improvements in RT delivery technology now allow for treatment with focal, high-dose radiation to the lesion in one to five sessions, sparing normal liver parenchyma and offering excellent local control with a limited toxicity profile (15-19).
While several options exist for liver metastatic disease, the present review summarizes developments in RT treatment options including conventional RT, SBRT, and RE.
Liver metastases
Metastatic tumors of the liver, especially in the Western world are far more common than primary liver tumors and account for 25% of metastases to solid organs (20). Liver metastases will occur in 30-35% of all cancers and in approximately 45-50% of GI tumors. For example, 4.5% to 24% of patients with newly diagnosed colorectal cancer (CRC) present with synchronous liver metastases and an additional 8.1% to 20% will develop metachronous disease (21). Liver metastases occur via spread through the portal vein, hepatic artery, or retrograde lymphatic permeation. Metastases to the liver are usually histologically carcinomas including adenocarcinomas, followed by squamous cell carcinomas, and neuroendocrine carcinomas with lung, colon, pancreas, breast, and stomach making up the most common primary tumor sites (22).
For patients who present with or develop metastatic disease, palliative systemic chemotherapy may be offered as an initial treatment with each regimen tailored to the primary site, though liver-related symptom relief is suboptimal (23). Local therapy can be administered with the goal of alleviating tumor-related symptoms, improving quality of life, and at times improving survival (6,7). Of note, not all patients diagnosed with liver metastases are precluded from a curative treatment. Select patients with limited oligometastatic disease can be offered tumor resection of the liver with a chance of long-term survival (2-5). The most studied and most commonly resected metastases are those of colorectal origin, with data showing five-year survival as high as 70% in patients with a solitary metastasis (24). Unfortunately, only 10-25% of patients with metastatic CRC are eligible for surgery secondary to comorbidities, extent of malignancy, or hepatic injury following chemotherapy (25).
Conventional radiotherapy
Conventional RT typically involves the use of photon irradiation delivered externally to a patient lying in the supine or prone position on a treatment table. These treatments were typically administered in the form of daily low-dose per fraction radiation (generally 1.8-2 Gy fractions) in an effort to avoid toxicity to the liver and nearby organs. This was historically performed without computed tomographic (CT) based 3D treatment planning until it became widely available. Radiation fields typically consisted of one anterior beam and one posterior beam. Organ motion management was not practiced. In the late 1970s, the Radiation Therapy Oncology Group (RTOG) conducted a prospective, non-randomized study exploring the use of radiation in the palliation of symptomatic liver metastases. In this study, patients received 30 Gy in 15 fractions (daily treatments), 25.6 Gy in 16 fractions, 20 Gy in 10 fractions, or 21 Gy in 7 fractions; additional radiation was allowed for patients with a solitary liver metastasis. Liver metastasis related pain was improved in 55% of patients with 40% experiencing partial normalizing of liver chemistries. While there were no cases of radiation-induced hepatitis, nephritis, or pneumonitis, nausea was aggravated in 16% of patients (1).
Other series from the same era also suggested a palliative benefit from RT for 50-95% of patients, though all of these utilized physician-reported outcomes as was standard for the time (1,9-12). Recently, a phase II trial of palliative RT for symptomatic liver tumors was conducted and utilized The Brief Pain Inventory (BPI), Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep), and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) to assess symptom relief (26). Patients were pretreated with oral granisetron 1 mg and oral dexamethasone 2 mg 1 hour before RT. In contrast to the previously mentioned studies, this group used a single fraction of 8 Gy, and CT based treatment planning. Radiation was administered to a large portion of, or the whole liver. At one month, 48% experienced symptom improvement. Improvements in hepatobiliary score, EORTC functional and symptom domains were also described. In addition, the treatment was well tolerated, with the most common treatment related side effect reported as transient grade 1 or 2 fatigue, while 7% of patients experienced grade 1 or 2 nausea. A single patient who declined premedication had grade 3 nausea and vomiting. Local control and disease free survival were not reported endpoints. Another prospective study evaluating short-course RT for symptomatic hepatic metastases treated partial liver or whole liver utilizing 10 Gy in 2 fractions (27). In this study, partial or complete symptomatic responses were achieved in 54% of patients though median survival was just ten weeks with all patients dying of progressive disease. Of note, at time of enrollment, 78% of patients had progressed on systemic therapy and 56% had ECOG 2 or 3 performance status. In these studies, approximately 50% of patients experienced some degree of pain relief though few achieved a complete or durable benefit. A randomized trial of short-course, palliative RT compared with best supportive care has been planned by the National Cancer Institute of Canada Clinical Trials Group (27).
For patients with lower disease burden in the liver and good performance status, treating a smaller volume of liver to a higher dose was found to offer improved local control and perhaps even survival benefit as compared with the doses used in the studies described above. This was achieved utilizing conformal RT, which leverages 3D treatment planning and on-treatment image guidance to direct dose to a specific treatment area within the liver. There are two techniques that have been used for administering conformal RT, 3D conformal radiotherapy (3DCRT), and intensity-modulated radiotherapy (IMRT). IMRT differs from 3DCRT in its ability to better limit dose to normal tissues and structures, though at a higher cost associated with treatment planning and delivery. Treatment of smaller volumes within the liver, as with these techniques, comes with special considerations which must be accounted for, particularly inter and intra-fraction liver motion.
A study treating colorectal metastases from the University of Michigan reported that approximately one-third of the liver can be irradiated to 72.6 Gy without significant toxicity when using a twice-daily fractionation regimen (28). Median survival was 20 months, representing a significant improvement from previous studies (1,9-12) reporting on whole liver RT. Similarly, another series from the same institution used three-dimensional conformal high dose RT to treat 128 patients, twice daily, with primary and metastatic liver cancer to a median dose of 60.7 Gy (29). Radiation dose was a significant predictor of survival, with patients receiving less than 60.7 Gy surviving a median 15.2 months compared with patients who received at least 60.7 Gy with a median survival of 18.4 months. Of the 103 evaluable patients, 11% had a complete response, 42% had a partial response, 45% had stable disease, and 3% had progressive disease. Thirty-one percent experienced at least a grade 3 toxicity including one treatment related death.
Patient selection
Conventional RT can be a reasonable treatment option for patients experiencing pain, nausea, vomiting, anorexia, or fatigue secondary to hepatic metastases. Pre-treatment liver function must be carefully evaluated in order to determine safety of RT and risk of RILD. For short-course, low dose RT, patients should have an international normalized ratio (INR) less than 3, platelet count greater than 25,000 per µm3, bilirubin less than 100 mol/L, and transaminases less than three times the normal limits. The Child-Pugh system scores laboratory and clinical factors to assess level of liver dysfunction (Table 1) and should be calculated for all patients prior to receiving RT. Patients with more severe baseline liver dysfunction as defined by Child-Pugh Class B or C, have less organ reserve and are at significantly higher risk of developing treatment-related liver problems (30,31). In one study, mean liver dose of 23 Gy was estimated to result in a 5% risk of liver toxicity for Child-Pugh Class A patients versus just 6 Gy for Child-Pugh Class B patients, emphasizing the importance of pretreatment evaluation (31). Caution should also be exercised in patients who are currently or have recently completed a course of systemic therapy or recent transarterial chemo-embolization (26). Further pre-treatment workup should be performed for patients being considered for dose-escalated conventional RT.

Full table
Summary
Palliation of symptoms related to metastatic disease is an important component in the management of patients with cancer spread to the liver. Conventional RT is a practical treatment option for liver metastases, however, there is no randomized evidence demonstrating that the efficacy and toxicity profile of this treatment is superior to other locoregional modalities. Short courses of RT including 8 Gy given in a single fraction or 10 Gy given in 2 fractions (administered with or without premedications) targeting large volumes may be considered in patients with poor performance status and significant burden of disease in the liver. These types of treatments offer patients the convenience of a single treatment and very low risk of treatment-related toxicity. Dose-escalated conventional RT offered improved local control and perhaps survival benefit in select patients with very limited metastatic burden but came with higher rates of GI toxicity. These treatment techniques set the stage for the application of SBRT to the treatment of liver metastases (to be described in the next section). While still offered at some facilities, dose-escalated conventional RT has largely been supplanted by SBRT in the treatment of inoperable oligometastatic or limited metastatic disease with good performance status.
Stereotactic body radiotherapy (SBRT)
SBRT, alternatively known as stereotactic ablative body radiotherapy (SABR), is a type of conformal external beam radiation therapy delivering large doses of radiation in five treatments or less as compared with conventional fractionation (where many small doses are generally delivered over a period of weeks). SBRT leverages advances in radiation treatment planning, respiratory (and therefore liver) motion management, and image guidance to allow for a very high level of accuracy (see Figure 1). Prior to a treatment planning appointment with the radiation oncologist, the patient may have radiopaque fiducial markers placed within the lesion by interventional radiology to assist in image-guidance and the radiation planning process. Lastly, the treatment planning process itself is typically more complex, often with images taken in all phases of the respiratory cycle. While SBRT is more costly and requires more intensive planning than conventional RT, this modality combines the local control benefit of dose-escalated fractionated RT with the convenience of short-course RT and an acceptable toxicity profile (15-19,32-35).
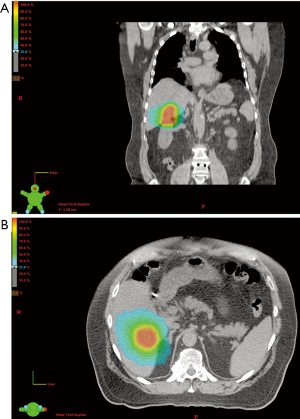
Much of the initial data describing the use of SBRT for liver tumors was in the management of unresectable hepatocellular carcinoma (HCC). The use of SBRT in the management of HCC was first described in 1995 (36). Since that time, SBRT has been associated with excellent local control rates (70-90% at 1-2 years), though mostly in patients with small, less than 6 cm tumors (37-39). From the published experiences with SBRT for HCC, valuable information regarding liver tolerance to RT at these high biologically effective doses was being established. In one study of 48 patients treated with 3-fraction liver SBRT (30-39 Gy), 11% of patients showed a post-treatment decline in Child-Pugh class. This was noted to be more likely if <800 cc of liver could be spared from doses at or exceeding 18 Gy (40).
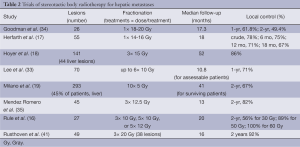
SBRT has more recently been studied in the setting of liver metastases. Treatment groups in reported series, however, have been relatively heterogeneous; most series include patients presenting with variable primary sites of disease, variable degrees of systemic control, and have often already failed some type of systemic and/or local therapy (15-19). Reported local control rates are variable, ranging from 50-93% and strongly correlates to treatment dose (Table 2).
Full table
In series that include both primary hepatocellular carcinoma and metastatic liver tumors, local control rates may be lower for metastatic tumors (15,42) than for primary liver tumors. In one series, one and 2-year local control rates were 93% and 93% for primary liver tumors versus 86% and 67% for CRC metastases (P=0.08) with all CRC failures lying within the radiation field, rather than marginal misses (15). In another study that included both primary and metastatic liver tumors, 1- and 2-year local control rates were reported at 90% and 55%; 9 local failures occurred at sites of liver-metastatic disease, 0 at sites of primary liver tumors (42). In this same study, 50% of CRC patients who received 30 Gy (10 Gy ×3 fractions) experienced local progression, whereas 0% (0/11) CRC patients treated with higher biologically effective doses of 36 Gy (12 Gy ×3 fractions) or 26 Gy (26 Gy ×1 fraction) experienced progression.
Given the described patterns of failure specific to liver metastases, often involving progression within the full-dose region, dose-escalated SBRT was investigated. In a phase I dose-escalation study of SBRT for liver metastases, complete plus partial response rate at one year was 90% in the 60 Gy group (12 Gy ×5 fractions) versus 30% in the 30 Gy group (10 Gy ×3 fractions), P=0.009 (16). In the experience from Rusthoven et al., local control following a high dose of 60 Gy (20 Gy ×3 fractions) was 94% at 2-year follow-up (41). Finally, in a recently published pool analysis, multivariable analysis demonstrated the significance of biologically effective dose, total dose, and dose per fraction as predictors of local control (43).
While it has been shown that SBRT administered with doses of 60 Gy (12 Gy ×5 fractions) is safe and effective, treated volumes must remain relatively small with the utmost respect for liver radiation tolerance (44) and the tolerance of the surrounding organs including large and small bowel, stomach, spinal cord, and kidney. For a 5-fraction regimen, Rule et al. limited at least 700 cc of liver to less than 21 Gy (16). Using this dose-constraint, no patients experienced any grade 2 or higher GI or hepatobiliary toxicities. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) recommends mean liver dose of less than 15 Gy when SBRT is administered in 3 fractions and that at least 700 cc of liver to less than 15 Gy (3-5 fractions) as per the study by Rusthoven et al. (14,41).
Patient selection
Patients requiring palliation of liver metastases should be offered SBRT when metastatic burden in the liver is low, in most studies defined as 3 or less lesions. While the gold standard for patients with oligmetastatic liver disease is surgery, SBRT is an excellent option for patients who are not operative candidates but local control may still be a priority. Of note, in these series, a small minority of the patients had tumors lying near the portal structures or stomach; the safety of treating lesions in these locations needs to be further characterized. A phase II trial is planned to investigate the most appropriate SBRT regimen for liver metastases utilizing a risk-adapted hypothesis model based on tumor location (16). While no absolute maximum tumor size has been established, 6cm has been used (41). Perhaps most importantly, treatment should allow for a significant portion of the uninvolved liver to receive limited integral dose as described above. Caution is advised for the treatment of patients with tumors lying near portal structures or near radiosensitive organs including stomach and small bowel or advanced Child-Pugh Class. Patients should not receive concurrent systemic therapy during SBRT outside the scope of a clinical trial. Patients included in the studies described were eligible for enrollment with total bilirubin less than 1.5 times the upper limit of normal, albumin at least 3 g/dL, transaminases less than 1.5 times the upper limit of normal, INR within normal range, hemoglobin of 10 g/dL, platelets 100,000 per µm3, and absolute neutrophil count of 1,000 per mm3 (16,44). Patients with centrally located tumors or with poor blood flow may be preferred candidates for SBRT over other therapies that may require adequate blood supply (RE and chemoembolization), more peripheral tumor location or distance from major vessels due to heatsink effect [cryoablation and radiofrequency ablation (RFA)].
Summary
SBRT is a safe and effective, noninvasive treatment option for patients with liver metastatic disease. Metastatic tumors appear to be more radioresistant than primary liver tumors and it has been shown that dose-escalation confers improved local control (15,42). A total of 60 Gy administered over the course of 5 fractions offers excellent local control and acceptable risk of toxicity if dose to uninvolved liver is limited, conferring long-term palliative potential. There are sufficient phase II and retrospective data to support the use of SBRT for patients with unresectable liver metastases and good performance status. Patients with moderate burden of liver-metastatic disease precluding the sparing of uninvolved liver with SBRT should be considered for RE.
Radioembolization (RE)
RE is a form of brachytherapy, which involves the direct intra-arterial delivery of radioactive isotopes close to or into a tumor. RE utilizing intravascular yttrium-90 microspheres has been shown to be a safe and efficacious modality in the treatment of unresectable primary and metastatic hepatic tumors (45-53). Other isotopes have also been used including iodine-131, rhemium-188, and holmium-166. While the majority of liver parenchyma is perfused via the portal venous system, hepatic tumors ≥3 mm in size receive 80-100% of their blood supply from the hepatic arterial system (54). This variation has been exploited to preferentially embolize tumors with chemotherapy, embolic agents, or radioactive microspheres delivering intra-arterial brachytherapy.
Microspheres embedded with yttrium-90 measure approximately 25-32 µm in diameter and are small enough to penetrate through tumor vasculature, but too large to pass through capillaries, avoiding migration into the cardiopulmonary system. Yttrium-90, beta emitter, decays to stable zirconium-90 with an average energy of 0.94 MeV, range of 1.1 cm, and a half-life of 64.2 hours (55). There are two yttrium-90 containing commercially available products: glass spheres (TheraSphere™, MDS Nordion, Ottowa, ON, Canada) approved by the Food and Drug Administration (FDA) in 1999 and resin spheres (SIR-Spheres®, Sirtex Medical, Sydney Cove, Australia), FDA approved in 2002.
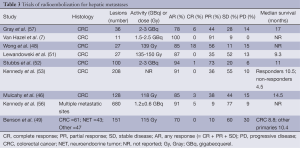
Clinical trials have established a benefit for RE with yttrium-90 microspheres conferring local control, progression free survival, and overall survival benefit in select patient populations. Any response rate (AR), defined as patients who had a complete response, partial response, or stable disease, approximates 79% in the salvage setting and 91% in the first-line setting (56). Reported median survival following yttrium-90 RE varies from 6.7-17 months (see Table 3). RE is well tolerated with minimal toxicity. Patients may experience postembolization syndrome, characterized by fatigue, nausea, abdominal pain, and/or a transient rise in liver function tests on the day of treatment to three days post-treatment (58). Common side effects include grade 2 fatigue (37%) and low-grade GI symptoms (25% including nausea, emesis, pain, and ulceration). Approximately 5% of patients may experience grade 3 GI symptoms. The incidence of post-RE RILD is <5% and the rates of radiation induced pancreatitis and pneumonitis are exceptionally low, at less than 1% (56).
Full table
In 2001, Gray et al. performed a randomized controlled trial with 76 patients treated with yttrium-90 RE prior to hepatic arterial infusion (HAI) of floxuridine versus HAI alone (57). The combination arm had a significantly improved tumor response and time to progression (TTP). The AR based on tumor volume was 78% in the combined arm compared to 59% in the HAI alone arm (P=0.03). The TTP was 15.9 versus 9.7 months in the combination and HAI arms respectively (P=0.001). Another randomized controlled trial by Van Hazel et al. in 2004 compared yttrium-90 microsphere RE plus systemic 5-fluorouricil (5-FU)/leucovorin versus 5-FU alone in 21 patients with unresectable liver metastases (7). The addition of yttrium-90 RE prior to chemotherapy was significantly associated with an improved AR rate (100% versus 60%; P<0.001), TTP (18.6 versus 3.6 months; P<0.0005), and survival (29.4 versus 12.8 months; P=0.02) compared to chemotherapy alone. Thirty-six months after randomization, 36% of patients in the combined therapy arm were alive compared to 0% of patients who received chemotherapy alone.
Multiple ongoing phase III trials are expected to add to the body of evidence supporting the use of yttrium-90 RE. The addition of RE to modern chemotherapy, specifically flurouracil, oxaliplatin, and leucovorin is the subject of two ongoing phase III trials. The first is an open-label randomized phase III trial of 5-Fluorouracil, OXaliplatin and Folinic acid, plus or minus Interventional Radio-Embolization as first line treatment for patients with unresectable liver-only or liver-predominant metastatic CRC (FOXFIRE; ISRCTN83867919). The second is a randomized controlled trial evaluating FOLFOX chemotherapy plus or minus SIR-Spheres for treatment of unresectable liver-only or liver-predominant CRC metastases (SIRFLOX; NCT00724503). The third, a phase III trial Evaluating TheraSphere in Patients with metastatic colorectal carcinoma Of the liver who have failed first line CHemotherapy (EPOCH; NCT01483027) randomizes patients who have failed first line therapy to receive standard second line chemotherapy with or without yttrium-90 RE and plans to accrue 360 patients.
Patient selection
The indications for RE include the presence of unresectable/inoperable primary or secondary liver malignancies in patients with liver-dominant tumor burden (although not necessarily exclusive to the liver). Patients with more than 3 liver lesions at high risk for developing further liver metastases may benefit from more extensive treatment with RE as compared with more focal modalities including SBRT, cryoablation, or RFA. Contraindications to this therapy (but not SBRT, cryoablation, or RFA) include inability to catheterize the hepatic artery, vascular shunting to lungs or uncorrectable evidence of reflux to GI organs on technetium-99m macro-aggregated albumin hepatic arterial perfusion scintigraphy (55). Patients should also have an ECOG performance status of 1 or KPS of 70 or more, and a life expectancy of at least three months. Further eligibility depends on multiple variables such as tumor burden in the liver, portal vein thrombosis, overall liver function including Child-Pugh Class, and prior radiation or chemotherapy.
Summary
RE with yttrium-90-labeled microspheres is a promising local treatment modality in the management of liver-metastatic disease. While phase III trials exploring RE as a first-line therapy for patients with liver metastases are ongoing, there are sufficient phase II and retrospective data to support its use as salvage therapy. Yttrium-90 RE should therefore be considered in patients with unresectable liver metastases, especially if disease burden does not allow for sufficient sparing of uninvolved liver with a less invasive SBRT technique.
Conclusions
Liver metastases are a common source of cancer-related morbidity. While systemic, palliative chemotherapy is an option for patients with significant disease burden, RT is a safe, well-tolerated local treatment that can offer durable tumor control and relief of symptoms. While a short course (1 or 2 fraction) of standard RT may be of benefit in palliating symptoms in patients with poor performance status and limited life expectancy, recent innovations in RT delivery, specifically SBRT and RE allow for durable local control of liver metastases in patients with longer life expectancy, limited metastatic disease burden, and good performance status. Randomized controlled trials with an emphasis on patient reported outcomes, quality of life assessment, and comparative effectiveness are needed to determine the best local treatment modality for patients with liver-metastatic disease. We recommend a multidisciplinary approach when weighing the risks and benefits of the available local treatment modalities for each patient.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Borgelt BB, Gelber R, Brady LW, et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys 1981;7:587-91. [PubMed]
- Fong Y, Blumgart LH, Cohen AM. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin 1995;45:50-62. [PubMed]
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [PubMed]
- Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11:1057-77. [PubMed]
- Amersi FF, McElrath-Garza A, Ahmad A, et al. Long-term survival after radiofrequency ablation of complex unresectable liver tumors. Arch Surg 2006;141:581-7; discussion 587-8. [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [PubMed]
- Wharton JT, Delclos L, Gallager S, et al. Radiation hepatitis induced by abdominal irradiation with the cobalt 60 moving strip technique. Am J Roentgenol Radium Ther Nucl Med 1973;117:73-80. [PubMed]
- Sherman DM, Weichselbaum R, Order SE, et al. Palliation of hepatic metastasis. Cancer 1978;41:2013-7. [PubMed]
- Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys 1977;2:129-32. [PubMed]
- Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA 1975;232:625-8. [PubMed]
- Leibel SA, Pajak TF, Massullo V, et al. A comparison of misonidazole sensitized radiation therapy to radiation therapy alone for the palliation of hepatic metastases: results of a Radiation Therapy Oncology Group randomized prospective trial. Int J Radiat Oncol Biol Phys 1987;13:1057-64. [PubMed]
- Austin-Seymour MM, Chen GT, Castro JR, et al. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys 1986;12:31-5. [PubMed]
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94-100. [PubMed]
- Liu E, Stenmark MH, Schipper MJ, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors. Transl Oncol 2013;6:442-6. [PubMed]
- Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081-7. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001;19:164-70. [PubMed]
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823-30. [PubMed]
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650-8. [PubMed]
- Abbruzzese JL, Abbruzzese MC, Lenzi R, et al. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 1995;13:2094-103. [PubMed]
- Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006;93:465-74. [PubMed]
- Centeno BA. Pathology of liver metastases. Cancer Control 2006;13:13-26. [PubMed]
- Blazeby JM, Fayers P, Conroy T, et al. Validation of the European Organization for Research and Treatment of Cancer QLQ-LMC21 questionnaire for assessment of patient-reported outcomes during treatment of colorectal liver metastases. Br J Surg 2009;96:291-8. [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [PubMed]
- Vauthey JN. Liver metastases. London: Springer, 2009.
- Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-6. [PubMed]
- Bydder S, Spry NA, Christie DR, et al. A prospective trial of short-fractionation radiotherapy for the palliation of liver metastases. Australas Radiol 2003;47:284-8. [PubMed]
- Robertson JM, Lawrence TS, Walker S, et al. The treatment of colorectal liver metastases with conformal radiation therapy and regional chemotherapy. Int J Radiat Oncol Biol Phys 1995;32:445-50. [PubMed]
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. [PubMed]
- Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys 2006;65:426-34. [PubMed]
- Xu ZY, Liang SX, Zhu J, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys 2006;65:189-95. [PubMed]
- Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol 2006;45:848-55. [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [PubMed]
- Mendez Romero A, Høyer M. Radiation therapy for liver metastases. Curr Opin Support Palliat Care 2012;6:97-102. [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [PubMed]
- Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010;10:475. [PubMed]
- Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209-14. [PubMed]
- Son SH, Choi BO, Ryu MR, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys 2010;78:1073-80. [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [PubMed]
- Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838-47. [PubMed]
- Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 2011;117:4060-9. [PubMed]
- Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005;62:1371-8. [PubMed]
- Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys 2009;74:1494-500. [PubMed]
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849-58. [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [PubMed]
- Wong CY, Salem R, Qing F, et al. Metabolic response after intraarterial 90Y-glass microsphere treatment for colorectal liver metastases: comparison of quantitative and visual analyses by 18F-FDG PET. J Nucl Med 2004;45:1892-7. [PubMed]
- Benson AB 3rd, Geschwind JF, Mulcahy MF, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional phase II study. Eur J Cancer 2013;49:3122-30. [PubMed]
- Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. [PubMed]
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol 2005;16:1641-51. [PubMed]
- Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg 2006;76:696-703. [PubMed]
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [PubMed]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954;30:969-77. [PubMed]
- ACR-ASTRO-SIR Practice Guideline for Radioembolization with Microsphere Brachytherapy Device (RMBD) for Treatment of Liver Malignancies 2008, Practice guideline.
- Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J 2010;16:163-75. [PubMed]
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [PubMed]


