Assessment and treatment of cancer pain: From Western to Eastern
Introduction
Pain is probably the most feared symptom in cancer patients. Unrelieved pain impacts all dimensions of quality of life and profoundly influences the patients' ability to endure treatment, return to health as a cancer survivor, or achieve a peaceful death. A recent review on cancer pain presented pooled prevalence rates from 33% after curative treatment to 64% in patients having advanced disease, with one-third overall rating their pain as moderate or severe (1). Despite significant medical, pharmacological and technological advances, the prevalence is still unacceptably high. Many treatment guidelines have been published during the past 20 years (2-7), and few data suggest that adherence to these guidelines yields satisfactory relief for cancer pain (8,9). These data warrant further study and professional education in this area.
This review will focus on the development in cancer pain classification and assessment, along with the available treatment approaches. It is hoped that increased awareness will lead to more comprehensive pain assessment and optimal pain management.
Cancer pain prevalence
The prevalence of cancer pain in the early reports ranged from 52% to 77% (10-13). More recent studies showed the figures that ranged from 24% to 60% in patients on active anticancer treatment (14-17) and 62-86% in patients with advanced cancer (18-22). Chronic pain was estimated to be approximately 33% in cancer survivor1. Even in the patients who have already received regular treatment, breakthrough pain rate was evaluated to be 89% (23), which have made the management more difficult. Such a high prevalence only illustrates that there are still great barriers to optimal assessment and management of cancer pain.
Classification of cancer pain
The palliative care population is heterogeneous in many different aspects, also in how pain is experienced and how it appears (24-26). Different pain characteristics depend on different etiological, pathological, physiological, anatomical and temporal factors. Also, psychological, cultural factors and other patient-related factors greatly impact the individual pain experience (27). Such a complexity has led to a challenge of how to systematically assess and classify all these factors to optimize the treatment of cancer pain.
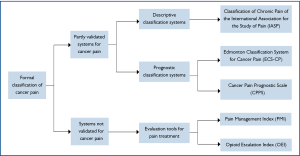
When reviewing the literatures, only three formal, systemati-cally developed and partially validated, pain classification systems were identified, namely the Classification of Chronic Pain of the International Association for the Study of Pain (IASP) (28), the Edmonton Classification System for Cancer Pain (ECS-CP) (29-32) and the Cancer Pain Prognostic Scale (CPPS) (33) (Figure 1).
Classification of chronic pain of the International Association for the Study of Pain (IASP)
The International Association for the Study of Pain (IASP) established a subcommittee on taxonomy in 1975 to achieve consensus on classification of chronic pain syndromes. A list of pain terms was first published in 1979 (34), later revised twice based on expert opinions and clinical experience (35). This is a descriptive coding system for chronic pain syndromes, both non-malignant and malignant, without any prognostication. Each clinical pain syndrome is assigned a code number based on five areas: anatomical site, organ systems whose abnormal functioning produces pain, temporal characteristics of pain, pain intensity and time since pain onset, pain etiology. This assessment is physician-based and consists of medical history, clinical examination and investigations. However, only one clinical study was identified which adopted the IASP Classification for Chronic Pain in cancer patients (28). To date, there is still no study to evaluate the clinical significance of IASP classification system.
Cancer Pain Prognostic Scale (CPPS)
The Cancer Pain Prognostic Scale (CPPS) was developed as a prognostic tool for prediction of pain relief in cancer patients. Four assessment instruments were used, all mainly based on patients' self-report: worst pain severity on an 11-point numerical rating scale (NRS) scale (0-10), emotional well-being from the Functional Assessment of Cancer Therapy (FACT-G), daily oral opioid dose of more than 60 mg and the presence of mixed pain. Similar to IASP classification system, there is only one study which adopted CPPS classification system (33).
Edmonton Classification System for Cancer Pain (ECS-CP)
The Edmonton Classification System for Cancer Pain (ECS-CP) is an instrument developed with the primary aim to predict response to treatment in patients with advanced cancer. It has gone through several stepwise and systematic validation studies, although its clinical application is still limited in Canada. In the first version of the ECS-CP (1989), named the Edmonton Staging System for Cancer Pain (ESS), patients with advanced diseases were categorized into three groups with good, intermediate or poor prognosis for pain treatment. This depended on their scores on seven domains: mechanism of pain, incident pain, previous narcotic exposure, cognitive function, psychological distress, opioid tolerance and history of drug or alcohol abuse (31). A subsequent study in 1995 led to a dichotomization of the groups, good or poor prognosis for pain control, because very few patients belonged to the group with intermediate prognosis (30). In addition, cognitive function and previous opioid consumption were removed from the staging system as they were identified to be unreliable factors for pain control (30).
In the subsequent multi-center trial which attempted to evaluate the reliability and predictive power of ESS, cognitive function was reintroduced based on expert opinions and literature reviews, while tolerance was excluded due to difficulty of clinical interpretation (32). Based on clinical experience and expert consensus (29) following a Delphi method consensus on construct validity (36), the rESS was renamed as a classification system (ECS-CP). Therefore, the current ECS-CP contains five domains: pain mechanism, incident pain, psychological distress, addictive behaviour and cognitive function.
In the view of above, only ECS-CP, out of other classification systems, has been employed in more than one study. Furthermore, ECS-CP is the only one classification tool that has been revised and updated with validation trials, expert opinions and consensus, and is now subject to a large international validation study which enrolls over 1,000 patients.
Assessment of cancer pain
A comprehensive assessment of cancer pain is the first important step toward optimal pain relief (37). Pain assessment should be brief, precise, multi-dimensional and specifically targeted to the patient population (27) (Table 1). However, at present there is no universally accepted tool to assess pain due to cancer in the palliative care setting (38,39). According to the recommendations on pain assessment in palliative care research from European Association for Palliative Care (EAPC), the selection of the instruments should be tailored to specific patient population and study design (40). For adult patients without cognitive impairment, Brief Pain Inventory short form (BPI-sf) (41), a multidimensional pain assessment tool should be given. The McGill Short Form questionnaire (42) is recommended for a more comprehensive pain assessment, such as studies focusing on diagnoses and characterization of various pain syndromes. On the other hand, for the assessment of pain intensity, a simple 11-point Numerical Rating Scales (NRS) is generally accepted.
Table 1
| Pain assessment should be based on Patient's self-report. | ||
|---|---|---|
| To characterize the pain experience | ||
| 1. | Intensity: at rest and with movements | |
| 2. | Location, referral and radiation pain | |
| 3. | Timing: onset, duration, course, persistent, or intermittent | |
| 4. | Quality: somatic pain, visceral pain, neuropathic pain | |
| 5. | Interference with quality of life: normal activities, psychological wellbeing, social and family relationship, sleep, appetite, sexual function, etc. | |
| 6. | Aggravating and relieving factors | |
| 7. | Associated symptoms | |
| 8. | Current management plan and its response | |
| Medical history | ||
| 1. | The extent of malignant disease, management plan, and prognosis | |
| 2. | Medical comorbidities | |
| 3. | psychiatric comorbidities | |
| 4. | Pre-existing chronic pain | |
| Physical examination | ||
| Essential laboratory and imaging investigations | ||
| To understand the nature of the pain and establish a "pain diagnosis" | ||
| 1. | Causes: cancer-related pain, treatment-related pain, pain unrelated to tumor or treatment | |
| 2. | Inferred pathophysiology: nociceptive, neuropathic | |
| Special issues that need to be concerned | ||
| 1. | Problems in communication and coordination | |
| 2. | Patient and family's beliefs on the pain experience and its management | |
| 3. | Patient and family's expectation and goal regarding pain relief | |
| 4. | The influence of cultural beliefs, religious and spiritual supports | |
| 5. | Psychological stress caused by pain and its treatment | |
| 6. | Financial burdens caused by pain and its treatment | |
But the problem is that the assessment of cancer pain is still far from satisfactory in cancer clinical practice, in spite of the recommendations mentioned above (38,43,44). Pain is still not routinely measured which may attribute to the fact that most systems are too long and cumbersome for patients and clinicians to use (43-49). Undoubtedly, a scientific and systematic approach to cancer pain assessment is necessary. However, the major problem with a multi-dimensional, specific tool is the increased burden on both patient and staff, which will in turn decrease its compliance and application.
Another issue which has not been addressed is that there is a continuous flow of novel assessment systems developed, though only few of them follow the standardized development procedures (50) (Table 2). According to a recent review which evaluated the content as well as the development and validation procedures of 11 pain assessment tools, only 2 instruments were fully validated or cross-culturally examined (50). Another review even reported that fewer that 3% of the studies had had their pain assessment packages tested or validated (51). In addition, the diversity of tools makes the comparison of results impractical. Therefore, we have realized that the continuous plethora of new tools is on the wrong track. What we need is a common language which could only be obtained by collaborative work. This will involve extensive literature review, expert opinions and consensus, rigorous translation procedures, and comprehensive validation (52). Only in this way, the standard of cancer pain assessment could be enhanced.
Table 2
| Assessment tools | Author, year | Dimensions | Items |
|---|---|---|---|
| Back Pain Function Scale (BPFS)* | Stratford, 2000 | Inf | 12 |
| Brief Pain Diary for ambulatory cancer care (BPD) | Maunsell, 2000 | Inf, Int, Treat | 8 |
| Migraine Disability Assessment Scale (MIDAS) | Stewart, 2000 | Dur, Inf, Int, Loc, Qual, Rel, Treat | 20 |
| Oswestry Disability Index 2 (OSW-2) | Cited in Roland, 2000 | Inf, Int | 10 |
| Expanded Prostate Cancer Index Composite (EPIC) | Wei, 2000 | Inf, Temp, Loc | 5 |
| M. D. Anderson Symptom Inventory (MDASI) | Cleeland, 2000 | Int | 1 |
| Cognitive Risk Profile (CRP) | DeGood, 2001 | Aff, Bel, Inf, Rel | 58 |
| Leeds assessment of neuropathic symptoms and signs (LANSS) | Bennett, 2001 | Qual, Temp | 5 |
| Numerical rating scales (NRS)* | Example in Turk, 2001 | Int | 1 |
| Pain Assessment Questionnaire for a patient with advanced disease (PAQ) | Perron, 2001 | Bel, Dur, Hist, Inf, Int, Loc, Qual, Rel, Temp, Treat | 14 |
| Verbal Rating Scales (VRS)* | Turk, 2001 | Int | 1 |
| Visual Analogue Scales (VAS)* | Example in Turk, 2001 | Int | 1 |
| Breast Cancer Treatment Outcomes Scale (BCTOS)* | Stanton, 2001 | Int | 3 |
| Edmonton Functional Assessment Tool-2 (EFAT-2)* | Kaasa, 2001 | Inf | 1 |
| Euro QOL Group (EQ-5D)* | Rabin, 2001 | Int | 1 |
| Cervical Spine Outcome Questionnaire (CSOQ) | BenDebba, 2002 | Inf, Int, Loc, Treat | 24 |
| Regional Pain Scale (RPS) | Wolfe, 2003 | Int, Loc | 38 |
| Medical Outcome Study 116 item core set (MOS-116)* | RAND, 2003 | Inf, Int, Temp | 8 |
| Pain assessment form (PAF) | Chen, 2003 | Int, Treat, Loc, Dur Bel | 5 |
| Pain Opioid Analgesics Beliefs Scale Cancer (POABS-CA) | Lai, 2003 | Bel | 10 |
| Resident Assessment Instrument for Palliative Care (RAI-PC) | Steel, 2003 | Int, Temp, Loc, Qual, Aff | 10 |
| World Health Organization Quality of Life Assessment Instrument ePain Module (WHQOL-Pain) | Mason, 2004 | Aff, Bel, Dur, Inf, Int, Loc, Temp, Treat | 28 |
| Medication Assessment Tool for Cancer Pain Management (MAT-PC) | Hakonsen, 2006 | Int, Treat, Loc, Qual, Dur | 34 |
| Korean Pain Assessment Tool (KCPAT) Patient Satisfaction Questionnaire | Choi, 2006 | Int, Loc, Qual, Aff, Treat, Inf | 5 |
Treat=Effects of treatment, Aff=Pain affect, Bel=Pain beliefs, Dur=Duration, Hist=Pain history, Inf=Pain interference, Int=Pain intensity, Loc=Pain location, Qual=Pain quality, Rel=Pain relief-exacerbating/relieving factors, TemP=Temporal pattern. * Unidimensional.
Treatment of cancer pain
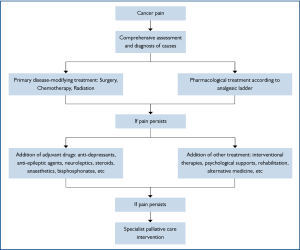
The management of cancer pain generally follows the flow chart in Figure 2. But now, it is highly recommended that it should be tailored to each individual, as different etiological, pathological, physiological, psychological, cultural, other patient-related factors or any combination of them altogether contribute to the complexity of cancer pain syndrome. While satisfactory pain relief never depends on a unique formula, the feasibility, appropriateness, potential benefits along with possible adverse effects of a treatment method should be taken into consideration in developing a coping strategy. The categories of treatment in cancer pain are listed in Table 3.
Table 3
| Primary disease-modifying treatment |
| • Palliative surgery |
| • Radiation |
| • Chemotherapy |
| Pharmacological treatment |
| • Opioid analgesics |
| • Non-opioid analgesics |
| • adjuvant analgesics |
| Interventional treatment |
| • Injection therapies |
| • Neural blockade |
| • Implant therapy |
| • Transcutaneous/ Transcranial neurostimulation |
| Mind-body approaches |
| • Rehabilitation |
| • Occupational therapy |
| • Hydrotherapy |
| • Psycho-educational interventions |
| • Cognitive behavioural therapy |
| • Relaxation therapy, guided imagery, other types of stress management |
| • Meditation |
| • Other forms of psychotherapy |
| Alternative treatment |
| • Acupuncture |
| • Massage |
| • Herbal |
| • Others |
Primary disease-modifying treatment
Approximately three quarters of cancer pain are directly related to tumor, while most of the remainder may attribute to anti-tumor treatment (53). Therefore, primary disease-modifying treatment plays an irreplaceable role in pain relief management. For instance, some metastasis lesions are usually restricted to bones, local compression and destruction are the major reasons for pain which could response to radiation effectively (54). Regarding chemotherapy, numerous confusing factors, like different regiments applied, have made it difficult to draw a positive conclusion. The decision of offering chemotherapy should depend on the clinical observation and balance between benefits and drawbacks.
Pharmacological treatment
Pharmacotherapy remains the single most important treatment option for the relief of cancer pain since the idea of analgesic ladder approach was raised in 1996 (55). Opioid analgesics, non-opioid analgesics and non-traditional analgesics were involved, of which opioid-based pharmacotherapy is considered to be the most effective.
Opioids
The representatives of opioid analgesics are morphine and codeine, which were also selected for the original WHO analgesic ladder (55). There are also many other opioids that have been permitted for the treatment of cancer pain (56). However, all these analgesics are subject to a range of side effects including tolerance, irritation, nausea and vomiting, constipation and even respiratory depression (57-62).
Morphine: Of all the opioid-based analgesics for treatment of cancer pain, morphine is still regarded as the golden standard, although its metabolite, M-3-G, may contribute to myoclonus, seizures, and hyperalgesia, particularly in patients with renal impairment (63). It is recommended for the severe pain intensity in the WHO analgesic ladder and comparison for other opioids (55). Morphine can be delivered in a wide range of formulations and routes, including oral, parenteral, and rectal delivery (64).
Codeine: Codeine or 3-methylmorphine is a relatively weak opioid which could be delivered alone, though it is usually combined with other pain killers like paracetamol. Codeine is less potent than morphine and has a correspondingly lower dependence-liability than morphine (65). It is considered a prodrug, since it is metabolisedin vivo to the primary active compounds morphine and codeine-6-glucuronide (C6G). This process depends mainly on an enzyme CYP2D6. Roughly 5-10% of codeine will be converted to morphine, with the remainder either free, conjugated to form codeine-6-glucuronide, or converted to norcodeine and hydromorphone (65,66). Because the different levels of CYP2D6 will lead to different capability of metabolism, it is estimated that 3% of Asians and African Americans and 10% of Caucasians are poor metabolizers for codeine which in turn causes reduced analgesic effect (67).
Fentanyl: Fentanyl is a potent synthetic narcotic analgesic with a rapid onset and short duration of action, which is approximately 100 times more potent than morphine (68,69), with 100 micrograms of fentanyl approximately equivalent to 10 mg of morphine and 75 mg of pethidine in analgesic activity. It can be administrated with a wide range of routes including parenteral, spinal, transdermal, transmucosal and rectal delivery (70,71). In cancer clinical practice, Fentanyl is frequently delivered with transdermal patches which slowly release the drug into the bloodstream over 48 to 72 hours, allowing for long-lasting relief from pain. Body temperature, skin type, amount of body fat, and placement of the patch may affect the rate of absorption (71). A recent study reported that the plasma level of fentanyl is lower in the cachectic cancer patients who received transdermal patches (69).
Oxycodone: Oxycodone is a new semi-synthetic opioid which was developed in an attempt to improve the existing opioids (72). It is effective for managing moderate to moderately severe acute or chronic pain (72), which can be administrated in immediate-release, long-lasting, and liquid formulations (73,74). In 2001, the European Association for Palliative Care recommended that oral oxycodone be a second-line alternative to oral morphine for cancer pain (75). Oxycodone can be delivered orally, intranasally, via intravenous/intramuscular/subcutaneous injection or rectally. The bioavailability of oral administration of oxycodone averages 60-87%, with rectal administration yielding the same results; intranasal varies between individuals with a mean of 46% (74).
Other opioids:There are still other opioids available for the treatment of cancer pain including buprenorphine, hydrocodone, hydromorphone, methadone, oxymorphone, tapentadol, tramadol, etc. However, pethidine and dextropropoxyphene are important exceptions due to the neurotoxic effects of their metabolites, normeperidine and norpropoxyphene, respectively (76). Levorphanol is the levorotatory stereoisomer of the synthetic morphinan and a pure opioid agonist, which is 4 to 8 times as potent as morphine and has a longer half-life (77). It has been applied rarely in the clinical practice probably because of its limited availability.
As mentioned above, satisfactory pain relief seldom depends on unique formula. Clinical observation and experience with opioid trials reflected the fact repeatedly that individuals responded differently to various opioids (78,79). While it is impractical to predict the most suitable opioid for individuals, the treatment of cancer pain could be initiated with any available opioid-based drugs and should be ready for switch.
Non-opioid and non-traditional analgesics
According to the WHO analgesic ladder, paracetamol and a non-steroidal anti-inflammatory drug (NSAID) is remmended for mild or moderate pain or as a supplement to opioid-based drug for severe pain (55).
Paracetamol: Paracetamol is a widely used over-the-counter analgesic and antipyretic. In recommended doses, the side effects of paracetamol are mild to non-existent, and therefore it is used frequently as a coanalgesic with opioid in order to enhance the efficacy (80,81). Recently, more attention has been drawn to its significant adverse side effects, especially the hepatic and renal impairment (82,83). This concern is even more intensive among the patients who is receiving chemotherapy, because there are case reports that interaction between anticancer agents and paracetamol aggravated hepatic toxicity (84). For the patients with impair renal or liver function and those with a history of alcohol abuse, paracetamol is now recommended to be avoided or the dosage should be limited within 2,000 mg each day (85).
NSAID: NSAIDs are usually indicated for the treatment of acute or chronic conditions where pain and inflammation are present, such as bone metastases. However, in the chronic cancer pain settings, safety concerns are always surrounding the administration of NSAIDs. The most potential adverse effects of NSAIDs include renal impairment, gastrointestinal upset, haematological and cardiovascular toxicity (86-91). The former three side effects are mainly caused by non-selective NSAIDs, like ibuprofen, which could inhibit the relevant enzymes to convert arachidonic acid to prostaglandins (86). On the other hand, the selective cyclo-oxygenase-2 inhibitors such as celecoxib have a potential risk of cardiovascular events including myocardial infarction and stroke (90,91). In view of the above, compromising function status of kidney or liver contraindicates the use of non-selective NSAIDs. In addition, NSAIDs should not be applied in the patients with platelet dysfunction or other bleeding disorders.
When serving as a supplement for opioid-based drugs, NSAIDs may offer the benefits of less nausea and vomiting, constipation and central nerve symptoms by reducing the dosage of opioid. More impressively, a recent systematic review has proven that the combination of non-opioid and opioid can even improve the efficacy of pain relief (92).
Non-traditional analgesics: When the patients with cancer pain poorly respond to opioids and/or non-opioid analgesics, their conditions may be managed by co-administration of non-traditional analgesics or adjuvant analgesics. These analgesics involve a wide range of medication, including anti-depressants, anti-epileptic agents, neuroleptics, steroids, anaesthetics, bisphosphonates, etc (93,94). Although the widespread use of these drugs as first-line agents in chronic non-malignant pain syndromes suggests that the term "adjuvant" is a misnomer, they usually are combined with a less-than-satisfactory opioid regimen when administered for cancer pain (93). Early use of anti-depressants as adjuvant analgesics is justified when pain is accompanied by depression (93). More importantly, based on their satisfactory relief for diverse types of neuropathic pain (95-98), the strongest indication for the use of anti-depressants occurs in the cancer patients with neuropathic pain who just partly response to opioids. Some severe pain caused by multifocal bone metastases which poorly responds to opioids could be addressed by corticosteroids in combination with bisphosphonates (99,100). Also, there is good evidence that anti-epileptic agents are valuable in treating refractory neuropathic pain (101,102), which may be relatively less responsive to opioids than other types of pain (103).
Other treatment for cancer pain
Apart from the mainstream of pharmaceutical therapy in the management of cancer pain, there remain various kinds of therapies which could serve as beneficial supplements or alternatives. These strategies include interventional therapies like neural blockage, rehabilitation like occupational therapy, psychological support like cognitive and behavioral therapy, and alternative therapies like acupuncture, massage and herbal medicine. Some modalities are even considered specifically for refractory cancer pain.
Interventional treatment
Interventional treatment consists of a large and varied group of injections, neural blockade approaches, implant therapies and neurostimulation (104-106). Spinal analgesics (intrathecal or epidural) provide significant hope for pain relief over months or years of treatment to help improve quality of life for an important minority of patients (2% to 5%) who suffer from severe and refractory cancer pain (104). Another example is coeliac plexus block for pain due to upper abdominal malignancy (105).
Mind-body approaches
Regarding other strategies involving psychological and rehabilitative therapies, they actually adopt the so-called mind-body approaches (107). They aim to address not only concurrent pain symptom but fatigue, sleep disturbance and other cumbersome symptoms as a common symptom cluster in patients with cancer. The relevant techniques include relaxation, meditation, imagery/hypnosis, music interventions and cognitive-behavioral therapy/coping skills training. It is evident that these therapies could help patients manage all the symptoms in the cluster with a single treatment strategy, and finally yield positive effects on broader quality of life (108,109).
Alternative treatment
Acupuncture: There has been an increasing trend of acupunc-ture research in the field of oncology in the past two decades, especially since the 1997 NIH consensus conference on acupuncture (110). More impressively, the number of randomized controlled trials (RCTs) also has risen significantly these years. A randomized, blinded, controlled trial demonstrated that auricular acupuncture was effective for neuropathic pain of various forms (111). Furthermore, it was also reported that acupuncture was helpful in the control of post-operative pain in patients with lung cancer, breast cancer, bladder cancer, prostate cancer, and ovarian cancer (112,113). While it seems promising particularly in the short term, there is still insufficient evidence to judge whether acupuncture is effective in treating cancer pain because of methodological limitations, small sample sizes, poor reporting and inadequate analysis. Further rigorous trials are required to obtain higher levels of evidence.
Massage: Massage is one of the oldest and most popular alternative interventions among cancer patients. It involves putting pressure and traction in the soft tissue in the body with therapeutic intent. It is not only an important part of Traditional Chinese Medicine, but also an important part of other types of complementary medical cultures, such as Indian medicine and reflexology. As massage seems to contribute to pain control through reduction of stress and anxiety levels (114), many studies have attempted to prove its efficacy and safety in cancer pain. A recent multi-institutional randomized controlled trial showed a favorable result that greater improvement in pain reduction was achieved in massage group, though it was criticized with its potential reporting bias (115). Unfortunately, most of the current trials regarding the value of massage in cancer pain treatment were subject to a variety of limitations including small sample size and lack of randomization, control or blinding methods (116). Therefore, further studies are warranted to avoid the deficiencies of previous researches.
Chinese herbal medicine: Chinese herbal medicine is mainly plant based, but some preparations include minerals or animal products. They can be packaged as powders, pastes, lotions or tablets, depending on the herb and its intended use. Different herbs have different properties and can balance particular parts of the body. Chinese herbal medicine, based on the theories of Traditional Chinese Medicine, has been used to treat neoplasm throughout the Chinese medical history. In the past two decades, an increasing number of clinical trials have tried to prove the role of herbs in cancer treatment and thus provide scientific evidence for clinical practice. A recent, systematic review regarding Chinese herbal medicine for cancer pain reported that 115 trials were included for analysis and 41 were labeled as randomized controlled trials, most of which displayed favorable outcome for herbs (117). However, these results should be interpreted with caution due to different herb formulas used, poor study designs and inadequate analysis. Another concern about Chinese herbal medicine is the relevant adverse effects and potential interaction with conventional medicine. The common adverse effects include skin rash, pruritus and blisters related to external application of herbal medicine; nausea, vomiting, dizziness and drowsiness when used orally; and fever, fatigue, dry mouth, skin rash with intravenous infusion. Severe side effects are uncommon but ever reported as neuromuscular symptoms like tremors in oral muscles and tongue numbness with oral preparations, and chest distress, dyspnea and arrhythmia with intravenous infusion. Most of the side effects are transient and self-limited, and do not require medical intervention. Studies have also suggested that the side effects of conventional analgesics could even be reduced when combined with the use of herbal medicine, which may thus enhance cancer patients' quality of life (117).
Although there is still far from enough evidence to obtain a clear picture regarding the benefits and the drawbacks of all the complementary strategies mentioned above, cancer centers offering comprehensive treatment options should provide access to these alternatives. They can be used when available, feasible, desired by the patient, and consistent with the goals of care (56).
Conclusions
Satisfactory pain relief remains the most important component in the palliative care of cancer patients. However, lack of common language and comprehensive validation in cancer pain classification and assessment has made it the single most critical barrier to optimal pain management. A standardized cancer pain assessment system should be established for both clinical practice and research purposes through international collaboration and consensus processes. Opioid-based pharmaceutical therapy is still the mainstream in the management of cancer pain, while more challenges have been arisen in the past 20 years. More attention has been thrown to other pharmaceutical therapies including non-opioid and adjuvant drugs, complementary therapies with mind-body approaches, alternative medicine or any scientific and experiential combination of them. Although there is still lack of evidence to support the current modalities which necessitates further studies, one thing is affirmed that cancer patients can expect a better quality of life in the future with advanced pain-relieving strategies integrated into palliative care.
Search strategy and selection criteria
We searched PubMed and references from relevant articles using the MeSH search terms "cancer pain", "palliative care", "assessment", "treatment" and "traditional Chinese medicine", and then by a more detailed search of PubMed for identified agents and methods. Papers published in English between Jan 1, 1960, and Sep 30, 2011, which discussed assessment and treatment of cancer pain were included.
Acknowledgements
This study was supported by the Youth Training Plan of Sun Yat-Sen University (No.10ykpy38), the Research Award Fund for Outstanding Young researchers in Sun Yat-sen Cancer Center (No.303045172006; No.303045172005), the National Natural Science Foundation of China (No.30901728), and the Science & Technology Pillar Program of Guangdong Province (No.2011B031800220).
References
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49.
- Jost L, Roila F. Management of cancer pain: ESMO clinical recommendations. Ann Oncol 2009;20:170-3.
- Cormie PJ, Nairn M, Welsh J. Control of pain in adults with cancer: summary of SIGN guidelines. BMJ 2008;337:a2154.
- Benedetti C, Brock C, Cleeland C, et al. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park) 2000;14:135-50.
- Dy SM, Asch SM, Naeim A, et al. Evidence-based standards for cancer pain management. J ClinOncol 2008;26:3879-85.
- Trescot AM. Review of the role of opioids in cancer pain. J NatlComprCancNetw 2010;8:1087-94.
- Green E, Zwaal C, Beals C, et al. Cancer-related pain management: a report of evidence-based recommendations to guide practice. Clin J Pain 2010;26:449-62.
- Azevedo São Leão Ferreira K, Kimura M, Jacobsen Teixeira M. The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer 2006;14:1086-93.
- Oldenmenger WH. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer 2009;45:1370-80.
- Sung JL, Wang TH, Yu JY. Clinical study on primary carcinoma of the liver in Taiwan. Am J Dig Dis 1967;12:1036-49.
- Lempinen M. Carcinoma of the stomach. I. Diagnostic considerations. Ann ChirGynaecolFenn 1971;60:135-40.
- Ross AP, Braasch JW, Warren KW. Carcinoma of the proximal bile ducts. SurgGynecolObstet 1973;136:923-8.
- Twycross RG. The terminal care of patients with lung cancer. Postgrad Med J 1973;49:732-7.
- Pignon T, Fernandez L, Ayasso S, et al. Impact of radiation oncology practice on pain: a cross-sectional survey. Int J RadiatOncolBiol Phys 2004;60:1204-10.
- Puts MT, Versloot J, Muller MJ, et al. The opinion on care of patients with cancer undergoing palliative treatment in day care. Ned TijdschrGeneeskd 2004;148:277-80.
- Rietman JS, Dijkstra PU, Debreczeni R, et al. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. DisabilRehabil 2004;26:78-84.
- Taylor KO. Morbidity associated with axillary surgery for breast cancer. ANZ J Surg 2004;74:314-7.
- Bradley N, Davis L, Chow E. Symptom distress in patients attending an outpatient palliative radiotherapy clinic. J Pain Symptom Manage 2005;30:123-31.
- Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer 2004;90:2288-96.
- Wilson KG, Graham ID, Viola RA, et al. Structured interview assessment of symptoms and concerns in palliative care. Can J Psychiatry 2004;49:350-8.
- Hwang SS, Chang VT, Cogswell J, et al. Study of unmet needs in symptomatic veterans with advanced cancer: incidence, independent predictors and unmet needs outcome model. J Pain Symptom Manage 2004;28:421-32.
- Strömgren AS, Groenvold M, Petersen MA, et al. Pain characteristics and treatment outcome for advanced cancer patients during the first week of specialized palliative care. J Pain Symptom Manage 2004;27:104-13.
- Zeppetella G, O'Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage 2000;20:87-92.
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999;353:1695-700.
- Strang P. Emotional and social aspects of cancer pain. ActaOncol 1992;31:323-6.
- Osipova NA, Novikov GA, Ziai GR, et al. Tramal in the treatment of acute and chronic pain syndromes in cancer patients. AnesteziolReanimatol 1990;6:55-8.
- Hjermstad MJ, Fainsinger R, Kaasa S. Assessment and classification of cancer pain. CurrOpin Support Palliat Care 2009;3:24-30.
- Grond S, Zech D, Diefenbach C, et al. Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain 1996;64:107-14.
- Nekolaichuk CL, Fainsinger RL, Lawlor PG. A validation study of a pain classification system for advanced cancer patients using content experts: the Edmonton Classification System for Cancer Pain. Palliat Med 2005;19:466-76.
- Bruera E, Schoeller T, Wenk R, et al. A prospective multicenter assessment of the Edmonton staging system for cancer pain. J Pain Symptom Manage 1995;10:348-55.
- Bruera E, MacMillan K, Hanson J, et al. The Edmonton staging system for cancer pain: preliminary report. Pain 1989;37:203-9.
- Fainsinger RL, Nekolaichuk CL, Lawlor PG, et al. A multicenter study of the revised Edmonton Staging System for classifying cancer pain in advanced cancer patients. J Pain Symptom Manage 2005;29:224-37.
- Hwang SS, Chang VT, Fairclough DL, et al. Development of a cancer pain prognostic scale. J Pain Symptom Manage 2002;24:366-78.
- Bonica JJ. The need of a taxonomy. Pain 1979;6:247-8.
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226.
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008-15.
- Portnow J, Lim C, Grossman SA. Assessment of pain caused by invasive procedures in cancer patients. J NatlComprCancNetw 2003;1:435-9.
- Caraceni A, Brunelli C, Martini C, et al. Cancer pain assessment in clinical trials. A review of the literature (1999-2002). J Pain Symptom Manage 2005;29:507-19.
- Carr DB, Goudas LC, Balk EM, et al. Evidence report on the treatment of pain in cancer patients. J Natl Cancer Inst Monogr 2004;32:23-31.
- Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage 2002;23:239-55.
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17:197-210.
- Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191-7.
- Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001;93:247-57.
- Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst Monogr 2004;32:9-16.
- Brescia FJ, Portenoy RK, Ryan M, et al. Pain, opioid use, and survival in hospitalized patients with advanced cancer. J ClinOncol 1992;10:149-55.
- Perron V, Schonwetter RS. Assessment and management of pain in palliative care patients. Cancer Control 2001;8:15-24.
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994;330:592-6.
- De Conno F, Caraceni A, Gamba A, et al. Pain measurement in cancer patients: a comparison of six methods. Pain 1994;57:161-6.
- Twycross R, Harcourt J, Bergl S. A survey of pain in patients with advanced cancer. J Pain Symptom Manage 1996;12:273-82.
- Hjermstad MJ, Gibbins J, Haugen DF, et al. Pain assessment tools in palliative care: an urgent need for consensus. Palliat Med 2008;22:895-903.
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain 2003;4:2-21.
- Kaasa S. Palliative care research: time to intensify international collaboration. Palliat Med 2008;22:301-2.
- Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain 1999;82:263-74.
- Falkmer U, Järhult J, Wersäll P, et al. A systematic overview of radiation therapy effects in skeletal metastases. ActaOncol 2003;42:620-33.
- McGrath PA. Development of the World Health Organization Guidelines on Cancer Pain Relief and Palliative Care in Children. J Pain Symptom Manage 1996;12:87-92.
- Portenoy RK. Treatment of cancer pain. Lancet 2011;377:2236-47.
- Dale O, Moksnes K, Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects. A systematic review. Palliat Med 2011;25:494-503.
- Boswell K, Kwong WJ, Kavanagh S. Burden of opioid-associated gastrointestinal side effects from clinical and economic perspectives: a systematic literature review. J Opioid Manag 2010;6:269-89.
- Gregorian RS Jr, Gasik A, Kwong WJ, et al. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain 2010;11:1095-108.
- Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med 2009;10:654-62.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008;11:S105-20.
- Berde C, Nurko S. Opioid side effects--mechanism-based therapy. N Engl J Med 2008;358:2400-2.
- Lötsch J. Opioid metabolites. Pain Symptom Manage 2005;29:S10-24.
- Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol 2010;11:484-9.
- Vree TB, van Dongen RT, Koopman-Kimenai PM. Codeine analgesia is due to codeine-6-glucuronide, not morphine. Int J ClinPract 2000;54:395-8.
- Srinivasan V, Wielbo D, Tebbett IR. Analgesic effects of codeine-6-glucuronide after intravenous administration. Eur J Pain 1997;1:185-90.
- Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007;7:257-65.
- Shibutani K, Inchiosa MA Jr, Sawada K, et al. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth 2005;95:377-83.
- Heiskanen T, Mätzke S, Haakana S, et al. Transdermal fentanyl in cachectic cancer patients. Pain 2009;144:218-22.
- Sathyan G, Jaskowiak J, Evashenk M, et al. Characterisation of the pharmacokinetics of the fentanyl HCl patient-controlled transdermal system (PCTS): effect of current magnitude and multiple-day dosing and comparison with IV fentanyl administration. ClinPharmacokinet 2005;44:7-15.
- Mystakidou K, Katsouda E, Tsilika E, et al. Transdermal therapeutic fentanyl-system (TTS-F). In Vivo 2004;18:633-42.
- Oxycodone Kalso E. J Pain Symptom Manage 2005;29:S47-56.
- Davis MP, Varga J, Dickerson D, et al. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer 2003;11:84-92.
- OrdóñezGallego A. González Barón M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. ClinTranslOncol 2007;9:298-307.
- Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 2001;84:587-93.
- Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol 1983;13:180-5.
- Prommer E. Levorphanol: the forgotten opioid. Support Care Cancer 2007;15:259-64.
- Fine PG, Portenoy RK. Establishing "best practices" for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009;38:418-25.
- Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage 2009;38:426-39.
- Hyllested M, Jones S, Pedersen JL, et al. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br J Anaesth 2002;88:199-214.
- Luyk N. Review of pain study of aspirin, ibuprofen and paracetamol. N Z Dent J 2000;96:66.
- Collins C, Starmer GA. A review of the hepatotoxicity of paracetamol at therapeutic or near-therapeutic dose levels, with particular reference to alcohol abusers. Drug Alcohol Rev 1995;14:63-79.
- Israel FJ, Parker G, Charles M, et al. Lack of benefit from paracetamol (acetaminophen) for palliative cancer patients requiring high-dose strong opioids: a randomized, double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage 2010;39:548-54.
- Ridruejo E, Cacchione R, Villamil AG, et al. Imatinib-induced fatal acute liver failure. World J Gastroenterol 2007;13:6608-111.
- Swarm R, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J NatlComprCancNetw 2010;8:1046-86.
- Rainsford KD. Anti-inflammatory drugs in the 21st century. SubcellBiochem 2007;42:3-27.
- Naesdal J, Brown K. NSAID-associated adverse effects and acid control aids to prevent them: a review of current treatment options. Drug Saf 2006;29:119-32.
- Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment PharmacolTher 2009;30:517-31.
- Innes JF, Clayton J, Lascelles BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec 2010;166:226-30.
- Krötz F, Struthmann L. A Review on the risk of myocardial infarction associated with the NSAID diclofenac. CardiovascHematolDisord Drug Targets 2010;10:53-65.
- Kerr DJ, Dunn JA, Langman MJ, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med 2007;357:360-9.
- McNicol E, Strassels SA, Goudas L, et al. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev 2005;CD005180.
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004;9:571-91.
- Gordon DB. Nonopioid and adjuvant analgesics in chronic pain management: strategies for effective use. NursClin North Am 2003;38:447-64. vi.
- Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60:1284-9.
- Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain 2002;6:17-24.
- Semenchuk MR, Davis B. Efficacy of sustained-release bupropion in neuropathic pain: an open-label study. Clin J Pain 2000;16:6-11.
- Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57:1583-8.
- Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat ClinPractOncol 2009;6:163-74.
- Vecht CJ, Haaxma-Reiche H, van Putten WL, et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology 1989;39:1255-7.
- Backonja MM. Anticonvulsants (antineuropathics) for neuropathic pain syndromes. Clin J Pain 2000;16:S67-72.
- Backonja MM. Use of anticonvulsants for treatment of neuropathic pain. Neurology 2002;59:S14-7.
- Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 2003;60:1524-34.
- Sloan PA. Neuraxial pain relief for intractable cancer pain. Curr Pain Headache Rep 2007;11:283-9.
- Brogan S, Junkins S. Interventional therapies for the management of cancer pain. J Support Oncol 2010;8:52-9.
- Tay W, Ho KY. The role of interventional therapies in cancer pain management. Ann Acad Med Singapore 2009;38:989-97.
- Kwekkeboom KL, Cherwin CH, Lee JW, et al. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage 2010;39:126-38.
- Cassileth BR, Keefe FJ. Integrative and behavioral approaches to the treatment of cancer-related neuropathic pain. Oncologist 2010;15:19-23.
- Bardia A, Barton DL, Prokop LJ, et al. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J ClinOncol 2006;24:5457-64.
- NIH Consensus Conference. Acupuncture. JAMA 1998;280:1518-24.
- Alimi D, Rubino C, Pichard-Léandri E, et al. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J ClinOncol 2003;21:4120-6.
- Wong RH, Lee TW, Sihoe AD, et al. Analgesic effect of electroacupuncture in postthoracotomy pain: a prospective randomized trial. Ann ThoracSurg 2006;81:2031-6.
- Mehling WE, Jacobs B, Acree M, et al. Symptom management with massage and acupuncture in postoperative cancer patients: a randomized controlled trial. J Pain Symptom Manage 2007;33:258-66.
- Corbin L. Safety and efficacy of massage therapy for patients with cancer. Cancer Control 2005;12:158-64.
- Kutner JS, Smith MC, Corbin L, et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med 2008;149:369-79.
- Ernst E. Massage therapy for cancer palliation and supportive care: a systematic review of randomised clinical trials. Support Care Cancer 2009;17:333-7.
- Xu L, Lao LX, Ge A, et al. Chinese herbal medicine for cancer pain. Integr Cancer Ther 2007;6:208-34.