Palliation of chronic obstructive pulmonary disease
Introduction
The objective of this review is to describe the physical and psychosocial symptoms associated with advanced chronic obstructive pulmonary disease (COPD) and to be able to identify and overcome barriers to providing needed palliative care to these patients and their families. Treatment options, both pharmacological and non-pharmacological for disease process as well as symptoms associated with advanced COPD will be discussed. We will identify psychosocial, spiritual and caregiver issues that are commonly seen in these patients. In addition, factors associated with poor prognosis will be identified. We will begin with a clinical case that highlights the topic of this review.
A case: Mr. J
Mr. J is a 76-year-old male Korean war veteran with a past medical history of very severe COPD (FEV1 =29) on home oxygen, coronary artery disease, diabetes mellitus, hypertension, tobacco abuse with 100 pack-year smoking history, alcohol abuse and post-traumatic stress disorder. He lives at home alone, and has a supportive daughter who visits him often. She supplies his meals and helps with chores around the house; however, she works full time and is a single mother. He has had three admissions to the hospital for COPD exacerbations within the last year, resulting in a decline of his functional status, now requiring a walker to ambulate which is restricted secondary to dyspnea on exertion. He has been losing weight with a decreased appetite.
The burdens of COPD
COPD is a leading cause of morbidity and mortality worldwide, expected to be the third leading cause of death by 2020 (1). Currently, COPD is the 4th leading cause of death in the United States, with female mortality doubling over the last 20 years (2). It is estimated that patients with COPD require 15 million visits per year, with 1.5 million emergency department (ED) visits. COPD results in 150 million days of disability per year, with the total direct cost per year being $29.5 billion, and indirect costs $20.4 billion (3). Patients with advanced COPD experience a progressive decline of functional status, FEV1 and symptom control . They are less likely to have involvement of expert in palliation despite their poor prognosis, morbidity and comparable mortality to lung cancer (4). Exacerbations and comorbidities contribute to the overall severity in individual patients (5). It is common for many patients to have concomitant depression or anxiety along with advanced COPD, as their activities of daily living can result in distress and existential suffering.
COPD is defined as a progressive disease state characterized by airflow limitation that is not fully reversible, and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases. The 2011 Global Strategy for the Diagnosis, Management and Prevention of COPD (GOLD) stratification of severity is based on post-bronchodilator forced expiratory volume in one second/forced vital capacity via spirometry, health status and exacerbations and dyspnea (6). Of the many risk factors including occupational exposure, socioeconomic status and genetic abnormalities, cigarette smoking is the most common cause of COPD worldwide (and is the reason why smoking cessation is a major goal in the management of COPD).
The World Health Organization defines palliative care as patient and family centered care that optimizes quality-of-life by anticipating, preventing and treating suffering. Palliative care throughout the continuum of illness involves addressing physical, intellectual, emotional, social and spiritual needs and to facilitate patient autonomy, access to information and choice (7). Patients who are under the care of a palliative care team are less likely to undergo aggressive care in the ICU, resulting in lower inpatient costs and improved quality-of-life. Patients who are offered palliative care are given an opportunity for communication between the patient, family and provider about their disease, how to cope, and what to expect as they get sicker. Given this opportunity, advanced directives can be addressed and patients are found to have increasing energy and understanding of their disease and prognosis (8).
Services provided by a palliative care team are valuable in the management of symptoms and progression of disease process as it focuses on quality of life, increasing function and reducing symptoms. Like many other advanced illnesses, COPD not only affects physical health, but also social and psychological health (9). Patients usually follow an overall downward trajectory of illness, one with worsening condition, followed by improvement in performance status with an overall progression towards complete disability, followed by death (10).
Barriers to palliative care
The discrepancies in palliative treatment for patients with COPD occur due to multiple barriers for caregivers, patients, and physicians. Physician barriers include: the lack of time, low levels of confidence with discussing prognosis in advanced COPD and limited resources. Patient barriers include the unwillingness to discuss end-of-life care, lack of communication, loss of hope, and poor of knowledge on types of care available (11-13). A study in New Zealand provided strategies to overcome some of these barriers; including the use of uncertainty to ease discussion, being careful and respectful, building relationships over time with patients, and beginning discussion about disease process and prognosis early in the course of disease (14).
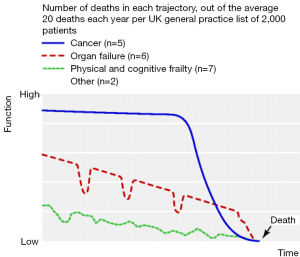
As a result of these barriers, patients with COPD often do not receive palliative care when compared to those with other advanced illnesses (15). One major reason why practitioners are hesitant to discuss end-of-life planning with their patients suffering from COPD is due to the challenge of prognostic accuracy (16). Unlike other chronic advanced illness such as lung cancer, COPD has a variable illness trajectory, making it difficult to predict prognosis (Figure 1).
Studies have shown that patients with COPD are less likely to receive palliative care services and are less likely to die at home compared to patients with lung cancer (4). Causes of death in patients with COPD include acute on chronic respiratory failure (30%), heart failure (13%), pulmonary infections, pulmonary embolism, cardiac arrhythmias and lung cancer (17).
Gore et al. studied 50 patients with COPD and 50 patients with inoperable non-small cell lung cancer (NSCLC) above the age of 60. This study found that patients with COPD had more depression, anxiety and scored worse on the Medical Outcome Study Short Form-36 Health Survey than patients with NSCLC. In addition, 82% of patients with COPD were housebound compared to 36% with lung cancer, yet the patients with COPD were not under the care of experts in palliative care medicine (4). Similar studies found that patients with lung cancer were more likely to receive hospice care (30%) than with patients with COPD (0%) (10).
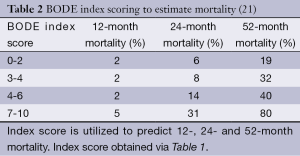
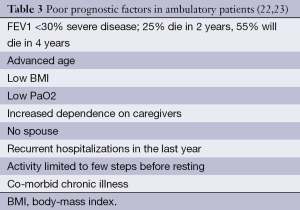
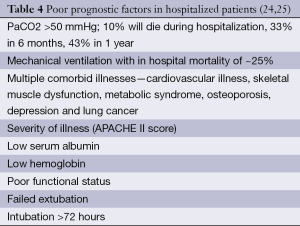
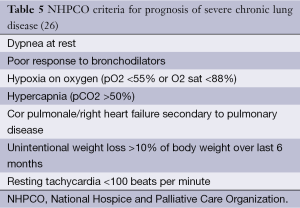
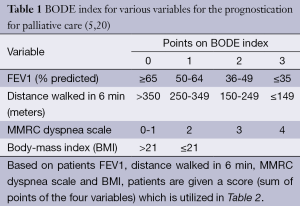
Assessment of severity of lung function impairment, frequency of exacerbations resulting in hospitalizations and the requirement for long-term oxygen therapy can help identify patients with more advanced disease (18,19). The BODE scale (Table 1) is frequently used in the prognostication of COPD, to help predict survival over 1-3 years (20) (Table 2). Several other prognostic factors should be taken into consideration while providing care for patients with COPD. Tables 3 and 4 discuss poor prognostic factors in ambulatory versus hospitalized patient, while Table 5 outlines the National Hospice & Palliative Care Organization Criteria for prognostication of severe chronic lung disease.
Full table
Case continues
Mr. J takes tiotropium, mometasone inhalers, an albuterol meter-dosed inhaler as needed and oxygen 2 liters around the clock. His sister is visiting from out of town and notices that he is having a hard time getting around, has been using his albuterol inhaler more frequently than normal, however, she doesn’t think he is using it properly. In addition, she feels that he is depressed, with feelings of hopelessness, worthlessness, insomnia, weight loss and poor appetite.
Treatment of COPD
Clinicians focus on treating COPD by using agents which reduce bronchoconstriction, airway inflammation and hyperinflation with pharmacotherapy (long-acting anticholinergics agents, with inhaled long-acting beta agonist and inhaled corticosteroids). As the disease a progress, supplemental oxygen therapy is often needed for hypoxemia as oxygen therapy improves survival in these cases. Oxygen therapy does not alter survival in patients with no hypoxemia (9).
Bronchodilators
Long-acting bronchodilators keep airways open to alleviate severe dyspnea. Anti-cholinergic such as tiotropium bromide have a long duration of action, selective for muscarine M1 and M3 receptors resulting in prolonged bronchodilation. This medication has been found to decrease COPD exacerbation frequency, improve quality-of-life and relieve dyspnea (27). It is important for patients to use the proper technique when using inhalers such as anticholinergics, LABA and inhaled corticosteroids.
Oral corticosteroids are not beneficial in the long term for chronic COPD. They may be beneficial in reducing airway inflammation during acute exacerbations, however, should not be used chronically due to the risk of steroid myopathy which may contribute to worsened lung function over time. In contrast, inhaled steroids can improve symptoms, lung function, quality-of-life and reduces the frequency of exacerbations in those with an FEV1 <60% predicted. However, they do not modify long-term decline of FEV1 or mortality, and withdrawal may cause exacerbations (28,29).
Methylxanthines such as theophylline or aminophylline are less effective and are less tolerated than inhaled long-acting bronchodilators. They act via stimulation of respiration centrally by enhancing sensitivity to the respiratory center to carbon dioxide. They increase biventricular systolic function therefore enhancing oxygen supply to respiratory muscles. However, they have a narrow therapeutic index (10-20 mcg/mL) as well multiple drug interactions limiting their use (29).
Patients who inadequately use inhalers should be taught proper techniques or should be offered nebulizers or spacers. This therapy is aimed at decreasing disease exacerbation, improving exercise tolerance and dyspnea. As COPD progresses, other non-pharmacological approaches are often needed to manage the patient. These include oxygen therapy and pulmonary rehabilitation.
Oxygen therapy
Oxygen therapy can be provided long term or short term. Long term oxygen therapy (LTOT) is administered for 12-15 hours per day and is indicated for hypoxemia (PaO2 55-59 mmHg), pulmonary hypertension, cor pulmonale and secondary polycythemia (9). Oxygen therapy is not benign however, as it can cause distress to a patient as their routines change, restrictions are placed on travel, and the perception of their illness by themselves and others change. LTOT can improve a patient’s health-related quality-of-life by lessening anxiety and depression but may also be limiting and intolerable by some (30). In addition, it has been shown improves survival, exercise, sleep and cognitive performance in hypoxemic patients (5).
Pulmonary rehabilitation
Pulmonary rehabilitation is defined by the National Institutes of Health Workshop on Pulmonary Rehabilitation Research as “a multidimensional continuum of services directed to persons with pulmonary disease and their families, usually by an interdisciplinary team of specialists, with the goal of achieving and maintaining the individual’s maximum level of independence and functioning in the community” (9). This approach improves symptom control, exercise tolerance and health related quality-of-life (31,32). Other non-pharmacological measures include pursed-lip breathing (expiration with pursed lips limits small airway collapse; decreases the respiratory rate (RR), work of inspiratory muscles and dyspnea resulting improved oxygenation), fans, relaxation techniques and paced activities. Although these methods may not affect disease progression, they have been shown to improve level of independence, functioning and quality-of-life (17).
Case continues
Mr. J’s daughter is concerned that he is not acting himself, lethargic, and slightly confused and she calls 911. He is subsequently brought to the ED and intubated for hypercapnic, hypoxic respiratory failure, develops a ventilator associated pneumonia for which he is successfully treated prior to extubation. On the general medical floor, he is still significantly dyspneic at rest, limiting his functional ability and is severely anxious.
Management of COPD among hospitalized patients
Important in the management of a patient with COPD, at any stage in the disease spectrum, is the discussion of advanced directives and goals of care. Without proper communication and documentation, patients’ wishes are unknown resulting in aggressive management at time of acute respiratory failure. Less than 15% of patients requiring intensive care will retain capacity to make decisions, making this a suboptimal time to discuss goals of care. The optimal time for this discussion should be early in the disease course as an outpatient, not after an exacerbation or hospitalization (33). Evidence shows that the presence of established advanced directives will provide decreased stress in the family and for the patient. In addition, it was associated with increased ratings of quality-of-care (34-36).
Physicians should explain the elevated in-hospital mortality rates, poor quality-of-life upon discharge, high frequency of short term readmission and poor prognosis with mechanical ventilation, so patients are well educated prior to making their decisions. Only one third of patients with oxygen-dependent COPD discussed end-of life-care with their physicians and less than 25% of doctors discussed important aspects of advanced illness (34,37). The SUPPORT trial (The Study to Understand Prognosis and Preferences for Outcomes and Treatments), found that patients with COPD were more likely to die in the ICU on mechanical ventilations and dyspnea than patients with lung cancer (38,39).
The management of acute or chronic respiratory failure can be managed be either noninvasive ventilation (NIV) or invasive ventilation with endotracheal tube, largely dependent on individuals’ advanced directives. Indications include acidosis (pH <7.35), despite optimal medical therapy and oxygen therapy, hypercapnia (PaCO2 greater than 45-60 mmHg), or RR greater than 24 breaths/min (5,40,41).
With the use of NIV, need for endotracheal intubation, complications of invasive intubation, hospital mortality and length of hospital stay has been shown to be reduced. A retrospective study in patients being treated with NIV for the first time showed that survival at 1, 2 and 5 years was 72%, 52% and 26% respectively (42,43). One year mortality was lower in patients receiving NIV for exacerbations of COPD, as compared to mechanical intubation and optimal medical therapy alone (5,30,44). Patients and family should be aware of the contraindications for NIV: respiratory arrest, cardiovascular instability, somnolence and altered mental status, copious secretions with aspiration risk, recent facial or gastroesophageal surgery, burns or extreme obesity. It is also is important for patients to understand that NIV should not be used routinely for palliation of dyspnea, as patients often continue to feel dyspneic while receiving the treatment (45).
Indications for intubation, should the patient request it, include severe acidosis (pH <7.25, hypercapnia, RR above 35 breaths/minute, life-threatening hypoxemia and/or NIV failure evidenced by worsening of arterial blood gases or pH in 1-2 hours (5). Patients who are intubated will have a poorer prognosis due to several factors, including respiratory muscle weakness, hypercapnia, hypoxia, age, malnutrition, and hemodynamic instability. These factors have been shown to contribute to prolonged intubation due to difficulty weaning (42).
Case continues
While on the general medical floor, the medical team asks for a palliative care consult. Mr. J and his visiting sister refuse to speak with the palliative care team because his pulmonologist recently told him he could “live a long time”. He still has dyspnea and depression, not adequately treated. He is discharged to a skilled nursing facility for inpatient rehabilitation.
Symptom management in COPD
Dyspnea is the most common symptom that patients experience with end-stage COPD. According to the United Kingdom (UK) regional study of care for the dying, 94% of patients with COPD complained of breathlessness, 67% of patients suffered from anorexia, and 44% had constipation. Another study found fatigue (71%), xerostomia (60%), cough (56%) anxiety (51%), drowsiness (47%), irritability (42%), pain (41%), and wheezing (40%) to be the most common symptoms in patients with advanced COPD (10). A prospective study of patients with end-stage COPD and lung cancer indicated that patients with COPD have significantly lower activities of daily living and physical, social and emotional functioning than patients with lung cancer (17). It has been shown that patients who survive acute COPD exacerbation requiring hospitalization will often have life-long dyspnea (46).
Dyspnea is described as the sensation of discomfort with breathing resulting in air hunger, increased work of breathing and chest tightness. The American Thoracic Society states that “dyspnea is a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, social and environmental factors, and may induce secondary physiological and behavioral responses” (9). Sensory signals from the respiratory system which include the upper airways, lungs and chest wall, are relayed to higher brain centers, specifically the limbic and paralimbic systems; where they are processed and influenced by behavioral, cognitive, contextual and environmental factors which contribute to the sensation of discomfort. Three key domains of breathlessness include perceived urge of the need to breathe, difficulty breathing and phase of respiration, and depth and frequency of breathing (9).
Case continues
After being in the rehabilitation facility for 10 days, Mr. J becomes lethargic and dyspneic. He is sent to the ED where he is found to have a pCO2 of 78. He maintains decision-making capacity and at this time refuses to be intubated. He is subsequently admitted to the ICU, placed on NIV, given steroids and antibiotics. However, he remains anxious and dyspneic, pulling at his BiPAP mask stating “stop torturing me, I just want to die”. A psychiatry consult is then placed, who recommend citalopram and deem that he is not at risk for suicide. In addition, a palliative care consultation is placed. Mr. J is now agreeable to using low dose opioids to alleviate dyspnea. He is started on 5 mg of oral liquid morphine every 4 hours along with every 2 hours as needed for dyspnea. Docusate and Sennakot are also prescribed to avoid opioid-induced constipation.
Opioids for refractory dyspnea
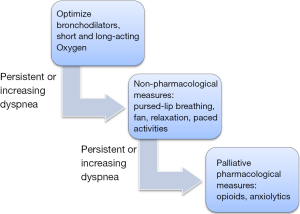
If dyspnea persists or increases despite above mentioned measures, it is considered refractory and opioids are recommended for palliation. The dyspnea ladder (Figure 2) can be referred to in the treatment of refractory dyspnea. Opioids alleviate dyspnea both endogenously and exogenously, centrally and peripherally and have a strong evidence to be effective for dyspnea (48-50). This is due to increased efficiency with exercise, reduction in the drive to breathe and reduced bronchoconstriction, therefore less hypoxia described as early as the 1960s (9). A crossover study reported improvements in sleep and refractory dyspnea with the use of 20 mg sustained release morphine daily (51-54).
Opioids for the treatment of refractory dyspnea are commonly not utilized to the full potential by medical providers due to concern of potential side effects such as respiratory depression and sedation (55). However, Uronis et al. found that morphine did not depress the RR [mean RR for morphine vs. placebo =20 (SD 5) vs. 21 (SD 4), P=0.143] and no episodes of sedation or obtundation were recorded (10). In fact, those using sustained released morphine reported better sleep and relief of breathlessness. A meta-analysis showed a statistically significant effect of opioids on breathlessness, with exercise tolerance as a secondary outcome (10). Clinical effect, measured by the visual analogue scale, was found to be relatively small and thought to be secondary to inappropriately small doses that were not titrated with prolonged dosing intervals (9). An Australian study examined 65 patients with refractory dyspnea who were treated with morphine SR (10-30 mg daily) and reported no cases of respiratory depression (32,56). The fear of respiratory depression when using opioids for dyspnea is a misconception, and usually does not happen if you start with a low dose and titrate up slowly while monitoring the patient. Physicians are commonly not comfortable and feel inexperienced with dosing, pharmacokinetics and titration of opioids.
In the treatment of opioid naïve patient, advice to “start low and go slow” can be given. There are also barriers to opioid prescription in regards to patient attitudes on morphine, nicknamed “opioid-phobia”. Thus it is important that the physician obtain the patients’ and their families trust by educating them in regards to treatment options for refractory dyspnea, as this will help them overcome their fear (57). Often patients may have a fear of addiction and a simple discussion explaining the difference between addiction and dependence usually improves understand and acceptance for trial. In addition, it is important for patient to understand that dyspnea may not be fully relieved, but that opioids are titrated with a goal to manage dyspnea so that it is tolerated by patient, which may vary from patient to patient.
Psychosocial and spiritual issues in COPD
Severe refractory dyspnea commonly results in anxiety symptoms and panic attacks which may worsen underlying dyspnea. Anxiety was found to be in 50% of patients with COPD (58,59). Low dose diazepam was initially utilized in the treatment of anxiety caused by and resulting in dyspnea however subsequent studies were unable to replicate these findings (9,60). A randomized controlled trial showed buspirone, a serotonergic anxiolytic agent, decreased dyspnea and exercise tolerance in COPD patients over a 2-week period. Anxiolytics have not been shown to benefit in the pathogenesis of dyspnea, however, help in anxiety which can perpetuate dyspnea (61).
The National Institute for Health and Care excellence (NICE) guideline recommends that patients who are found to be depressed or anxious should be treated with antidepressants. Surprisingly, 40-50% of patients with COPD are found to have depression, which is often untreated and is an independent predictor of mortality (58,59). Along with pharmacotherapy, counselling patients about the importance of treatment of underlying depression for management of COPD is beneficial. Treatment of depression, with counselling and pharmacotherapy, actually has shown to decrease the ratings of dyspnea and other physical symptoms (62). In one study, only one third of patients with COPD and clinically significant depression or anxiety were being treated and only half of those with severe depression were receiving treatment (63). “Depression was linked to deterioration in physical capacity which caused a decline in mobility, an inability to get out of the home and loss of a previously active person” (63). Not only do patients feel better with treatment, they are able to cope better and tolerate physical symptoms, such as breathlessness.
Functional capacity decreases as the disease progresses, resulting in increasing psychosocial needs, with reliance on caregivers. Patients become more dependent on family members and caregivers for activities of daily living, resulting in loss of independence. One caregiver reported “by the time we’d got her to the doctor’s office she’d be fighting for her breath even if she was sitting in the car” (8,64,65). Patients with COPD fear being a burden on their family, although there are few studies that measure caregiver burden (66). Caregiver burden, defined as “the strain or load borne by a person who cares for an elderly, chronically ill, or disabled family member or other person” can be divided into objective versus subjective. Objective burden involves the time spent, tasks performed and possible financial problems. Subjective burden includes physical, psychological, social and emotional impact experienced by those giving care (67).
A point will come in which leaving the house becomes difficult for the patient, proving home-based community services (nurses, respiratory therapists and physicians) to be beneficial in patients’ care. A randomized controlled trial found a team, home-based care approach reduced caregiver burden and improved quality-of-life (68). Patients with end-stage COPD, eligible for hospice care can benefit from home care which includes visiting home nurses, physicians, nurse aides, and medical appliances (i.e., hospital bed, home oxygen), medication, and supplies provided in the home. Social workers, chaplains, dieticians, physical and occupational therapists may also make visits in the home. Trained volunteers for patient companionship and caregiver respite are also available.
Case continues
The next day, Mr. J’s dyspnea improves, he is more alert and the primary team is able to remove his BiPAP. At this point Mr. J agrees to be transferred to the palliative care unit. There, his morphine requirements are titrated up to 10 mg every 4 hours with no adverse effects. He is now able to get out of bed and walk short distances with a walker. After discussion about goals of care, Jim agrees to a “do not resuscitate” and elects for no intubation, feeding tubes, invasive procedures or transfers to ICU. He designates his daughter as his first health care proxy and his sister as his second health care proxy. At this point, Mr. J wants to be discharged home, and his daughter agrees for him to live with her. Home hospice referral is made and the patient’s wishes are respected. Jim peacefully dies at home 2 weeks later with his family by his side and the assistance of home hospice. He was comfortable.
Conclusions
COPD is a complex and often unpredictable disease associated with multiple comorbidities and as it advances becomes burdensome. Physicians should always consider offering palliative care and inform patients of the nature and prognosis of the disease and listening to their needs, life values, and wishes. These discussions allow patients to make informed choices and help ensure that they receive end-of-life care consistent with their goals. Palliative care benefits patients through all stages of COPD because of patients’ high symptom burden that reduces physical, psychological, and social functioning. Dyspnea is the most common and distressing symptom in COPD patients, which responds only partially to therapy and eventually becomes refractory to routine care, which requires a shift from therapeutic goals of prolonging survival to palliative goals of relieving symptoms, improving function, and enhancing quality of life.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269-76. [PubMed]
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ 2002;51:1-16. [PubMed]
- Au DH, Udris EM, Fihn SD, et al. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med 2006;166:326-31. [PubMed]
- Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000;55:1000-6. [PubMed]
- Global initiative for chronic obstructive lung disease 2011: Global strategy for the diagnosis, management and prevention of COPD. Available online: http://goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- The National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care 3rd edition 2013. Available online: https://www.hpna.org/multimedia/NCP_Clinical_Practice_Guidelines_3rd_Edition.pdf
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2007;5:17. [PubMed]
- Elkington H, White P, Addington-Hall J, et al. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004;98:439-45. [PubMed]
- Uronis HE, Currow DC, Abernethy AP. Palliative management of refractory dyspnea in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:289-304. [PubMed]
- Brazil K, Bédard M, Krueger P, et al. Barriers to providing palliative care in long-term care facilities. Can Fam Physician 2006;52:472-3. [PubMed]
- Elkington H, White P, Higgs R, et al. GPs’ views of discussions of prognosis in severe COPD. Fam Pract 2001;18:440-4. [PubMed]
- Halliwell J, Mulcahy P, Buetow S, et al. GP discussion of prognosis with patients with severe chronic obstructive pulmonary disease: a qualitative study. Br J Gen Pract 2004;54:904-8. [PubMed]
- Schroedl C, Yount S, Szmuilowicz E, et al. Outpatient Palliative Care for Chronic Obstructive Pulmonary Disease: A Case Series. J Palliat Med 2014. [Epub ahead of print]. [PubMed]
- Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012;141:726-35. [PubMed]
- Seamark DA, Seamark CJ, Halpin DM. Palliative care in chronic obstructive pulmonary disease: a review for clinicians. J R Soc Med 2007;100:225-33. [PubMed]
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012;141:1216-23. [PubMed]
- Horton R, Rocker G, Dale A, et al. Implementing a palliative care trial in advanced COPD: a feasibility assessment (the COPD IMPACT study). J Palliat Med 2013;16:67-73. [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [PubMed]
- Roberts MH, Mapel DW, Bruse S, et al. Development of a modified BODE index as a mortality risk measure among older adults with and without chronic obstructive pulmonary disease. Am J Epidemiol 2013;178:1150-60. [PubMed]
- Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care 2004;49:90-7; discussion 97-8. [PubMed]
- Duenk RG, Heijdra Y, Verhagen SC, et al. PROLONG: a cluster controlled trial to examine identification of patients with COPD with poor prognosis and implementation of proactive palliative care. BMC Pulm Med 2014;14:54. [PubMed]
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67. [PubMed]
- Nevins ML, Epstein SK. Predictors of outcome for patients with COPD requiring invasive mechanical ventilation. Chest 2001;119:1840-9. [PubMed]
- Fox E, Landrum-McNiff K, Zhong Z, et al. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. JAMA 1999;282:1638-45. [PubMed]
- Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta-analysis of clinically relevant outcomes. Respir Care 2011;56:477-87. [PubMed]
- Cope S, Donohue JF, Jansen JP, et al. Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis. Respir Res 2013;14:100. [PubMed]
- Karbasi-Afshar R, Aslani J, Ghanei M. Efficacy and safety of inhaler steroids in COPD patients: Systematic review and meta-analysis of randomized placebo-controlled trials. Caspian J Intern Med 2014;5:130-6. [PubMed]
- Eaton T, Garrett JE, Young P, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J 2002;20:306-12. [PubMed]
- Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax 2009;64:910-5. [PubMed]
- Janssen DJ, McCormick JR. Palliative care and pulmonary rehabilitation. Clin Chest Med 2014;35:411-21. [PubMed]
- Curtis JR, Engelberg RA, Nielsen EL, et al. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J 2004;24:200-5. [PubMed]
- Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J 2008;32:796-803. [PubMed]
- Tilden VP, Tolle SW, Drach LL, et al. Out-of-hospital death: advance care planning, decedent symptoms, and caregiver burden. J Am Geriatr Soc 2004;52:532-9. [PubMed]
- Norris K, Merriman MP, Curtis JR, et al. Next of kin perspectives on the experience of end-of-life care in a community setting. J Palliat Med 2007;10:1101-15. [PubMed]
- White DB, Braddock CH 3rd, Bereknyei S, et al. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med 2007;167:461-7. [PubMed]
- Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc 2000;48:S146-53. [PubMed]
- Weingaertner V, Scheve C, Gerdes V, et al. Breathlessness, Functional Status, Distress, and Palliative Care Needs Over Time in Patients With Advanced Chronic Obstructive Pulmonary Disease or Lung Cancer: A Cohort Study. J Pain Symptom Manage 2014;48:569-81.e1.
- Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 2002;28:1701-7. [PubMed]
- Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis 2014;9:837-52. [PubMed]
- Carlucci A, Guerrieri A, Nava S. Palliative care in COPD patients: is it only an end-of-life issue? Eur Respir Rev 2012;21:347-54. [PubMed]
- Chung LP, Winship P, Phung S, et al. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology 2010;15:1084-91. [PubMed]
- Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax 2001;56:708-12. [PubMed]
- Curtis JR, Cook DJ, Sinuff T, et al. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007;35:932-9. [PubMed]
- Dahlin C. It takes my breath away: end-stage COPD. Part 1: a case study and an overview of COPD. Home Healthc Nurse 2006;24:148-55. [PubMed]
- Rocker GM, Sinuff T, Horton R, et al. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med 2007;10:783-97. [PubMed]
- Jennings AL, Davies AN, Higgins JP, et al. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev 2001;CD002066. [PubMed]
- Thomas JR, von Gunten CF. Clinical management of dyspnoea. Lancet Oncol 2002;3:223-8. [PubMed]
- Jennings AL, Davies AN, Higgins JP, et al. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002;57:939-44. [PubMed]
- Abernethy AP, Currow DC, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003;327:523-8. [PubMed]
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010;16:150-4. [PubMed]
- Currow D, Johnson M, White P, et al. Evidence-based intervention for chronic refractory breathlessness: practical therapies that make a difference. Br J Gen Pract 2013;63:609-10. [PubMed]
- Bausewein C, Simon ST. Inhaled nebulized and intranasal opioids for the relief of breathlessness. Curr Opin Support Palliat Care 2014;8:208-12. [PubMed]
- Hadjiphilippou S, Odogwu SE, Dand P. Doctors’ attitudes towards prescribing opioids for refractory dyspnoea: a single-centred study. BMJ Support Palliat Care 2014. [Epub ahead of print].
- Currow DC, Ekstrom M, Abernethy AP. Opioids for chronic refractory breathlessness: right patient, right route? Drugs 2014;74:1-6. [PubMed]
- Young J, Donahue M, Farquhar M, et al. Using opioids to treat dyspnea in advanced COPD: attitudes and experiences of family physicians and respiratory therapists. Can Fam Physician 2012;58:e401-7. [PubMed]
- Rocker GM, Sinuff T, Horton R, et al. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med 2007;10:783-97. [PubMed]
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23:345-9. [PubMed]
- Freeman D, Price D. ABC of chronic obstructive pulmonary disease. Primary care and palliative care. BMJ 2006;333:188-90. [PubMed]
- Lou P, Zhu Y, Chen P, et al. Interaction of depressive and anxiety symptoms on the mortality of patients with COPD: a preliminary study. COPD 2014;11:444-50. [PubMed]
- Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics 1992;33:190-201. [PubMed]
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005;127:1205-11. [PubMed]
- Addington-Hall J, Walker L, Jones C, et al. A randomised controlled trial of postal versus interviewer administration of a questionnaire measuring satisfaction with, and use of, services received in the year before death. J Epidemiol Community Health 1998;52:802-7. [PubMed]
- Sautter JM, Tulsky JA, Johnson KS, et al. Caregiver experience during advanced chronic illness and last year of life. J Am Geriatr Soc 2014;62:1082-90. [PubMed]
- Rocker GM, Sinuff T, Horton R, et al. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med 2007;10:783-97. [PubMed]
- Simpson AC, Young J, Donahue M, et al. A day at a time: caregiving on the edge in advanced COPD. Int J Chron Obstruct Pulmon Dis 2010;5:141-51. [PubMed]
- Hughes SL, Weaver FM, Giobbie-Hurder A, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA 2000;284:2877-85. [PubMed]
- Boland J, Owen J, Ainscough R, et al. Developing a service for patients with very severe chronic obstructive pulmonary disease (COPD) within resources. BMJ Support Palliat Care 2013. [Epub ahead of print].