Efficacy of granisetron and aprepitant in a patient who failed ondansetron in the prophylaxis of radiation induced nausea and vomiting: a case report
Background
Radiation therapy is an effective method of relieving pain caused by bone metastases (BM) and can significantly improve functional ability and decrease the need for analgesic use. While radiation therapy is an effective tool for relieving pain, it may also induce emesis in certain patients who receive it to more sensitive areas (1). Radiotherapy-induced nausea and vomiting (RINV) can severely debilitate a patient during and after treatment, which can lead to a decrease in quality of life (QOL) (1-3). RINV may also impact a patient’s ability to complete (4) or desire to accept further treatment, which may have undesirable disease outcomes and additional associated costs (1,3). Therefore, it is essential that RINV is prevented in order to avoid further compromise on QOL, functional abilities, and psychological status in palliative cancer patients (1-3).
Antiemetics, such as 5-hydroxytryptamine3 receptor antagonists (5-HT3 RAs), have been well validated for use with chemotherapy, yet few studies have been undertaken in the setting of radiation treatment. Current guidelines from the Multinational Association of Supportive Care Cancer (MASCC), European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO), and National Comprehensive Cancer Network (NCCN) recommend the routine use of 5-HT3 RAs for patients receiving moderate or highly emetogenic radiotherapy with the option to add dexamethasone in the latter situation (5). Despite the inclusion of recommendations for antiemetic agents in patients at risk for RINV within these documents, three of the four guidelines do not provide specific dosing information. Additionally, all sources noted a deficiency of evidence-based medicine exploring the mechanisms of RINV and effectiveness of antiemetics for RINV (1).
The mechanism of RINV is assumed to be similar to the one underlying chemotherapy-induced nausea and vomiting (CINV), and thus the vast amount of research performed in chemotherapy, as well as the most efficacious antiemetic medications for CINV, guide practice and recommendations in radiation (1). In most circumstances, a patient experiencing CINV on an antiemetic regimen will still respond to another antiemetic, including one from the same class (2). de Wit et al. (2) determined that if a patient fails on one 5-HT3 antagonist, the patient may still be offered a different agent in this class resulting in successful emesis control for CINV. The exact reasons for this observed phenomenon are not entirely clear and possible explanations include differences in metabolism, dosing, duration of action, ability to inhibit serotonin release from the GI tract and enterochromaffin cells, and genetic polymorphic differences in the serotonin reuptake transporter (2). For example, granisetron has a longer plasma half-life and duration than ondansetron; therefore, granisetron seems to have increased effectiveness in comparison to ondansetron (6). A study by Wu and Liaw (7) added aprepitant to patients who experienced acute or delayed emesis with 5-HT3 RAs and dexamethasone. The addition of apprepitant provided adequate emesis protection and was an alternative for patients who failed on typical prophylactic measures (7). This phenomenon has not yet been well studied in the radiation field, yet was trialed with this patient and yielded promising results.
Case presentation
A 47-year-old female with breast cancer and known extensive BM was referred in May 2014 with severe back pain. A subsequent MRI was performed and showed extensive lesions in the spinal vertebrae, pelvis, and bilateral hips and femurs. To decrease her back pain, she was recommended to undergo a course of radiation therapy to the lumbar spine, at a dose of 30 Gy in ten fractions. The patient was prescribed ondansetron 8 mg twice daily for the duration of treatment as an antiemetic.
Case management
The patient experienced severe nausea and vomiting on the first two days of radiation treatment. The treatment was halted until the patient recovered and was reassessed by the attending radiation oncologist and pharmacist, who then prescribed oral granisetron 2 mg once per day and oral aprepitant 125 mg for the first day, followed by 80 mg per day on the following two days for RINV. The patient was restarted on radiation treatment after the emesis resolved and she experienced no further vomiting, only intermittent nausea; however, the patient became severely drowsy and fatigued. The patient was given the option of continuing the current regime, replacing the aprepitant with dexamethasone, adding dexamethasone to the regime to curb drowsiness, or decreasing the frequency of aprepitant. As the patient previously had a negative experience with dexamethasone and was not keen on pursuing a triple agent regime, dexamethasone was excluded in this case; however, it may present a feasible and effective option in other circumstances. Further clinical discussions ensued and the dosing schedule for the aprepitant was changed to 125 mg for the first day and 80 mg every other day while at the same time continuing daily with the aforementioned granisetron dosage.
Case outcome
The frequency change that was implemented with the aprepitant resulted in lesser sedation, but there was no improvement in her mild nausea. The patient also experienced mild diarrhea on the final day of treatment and the subsequent three days. Radiation treatment was completed with no further emesis or severe nausea once switched to the granisetron and aprepitant regimen (Figure 1).
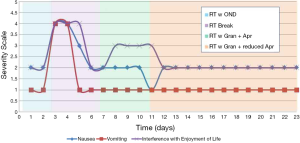
Conclusions
Based on the above case report, it appears that granisetron and aprepitant are efficacious anti-emetic agents in individuals who experience RINV and have failed RINV prophylaxis with ondansetron. It is essential that patients are closely monitored for RINV during treatment, as it can significantly impact their QOL and adherence to future radiotherapy. Physicians should be aware of other possible pharmaceutical options for the treatment of RINV if a patient fails on ondansetron.
Future studies should consider the effectiveness of palonosetron for RINV prophylaxis in patients still experiencing nausea and vomiting on available antiemetic therapies. Popovic et al. (8) conducted a review of sixteen randomized control trials that investigated a newer generation 5-HT3 RA called palonosetron. Their review demonstrated that palonosetron consistently shows superior nausea and emesis control in both acute and delayed phases in chemotherapy when compared to both ondansetron and granisetron (8). Furthermore, the authors concluded that palonosetron was safer to use and on most occasions required less rescue medications (8). Studies comparing the efficacy of 5-HT3 RAs such as granisetron and ondansetron have concluded that both antiemetics have no statistical significant differences in efficacy and result in similar emetic control (5). Dexamethasone in addition to a 5-HT3 RA was observed to have superior nausea and vomiting control than use of a 5-HT3 RA alone (5), yet was withheld in this circumstance due to patient preference. Herein, we presented the case of a patient who had substantially reduced nausea and complete resolution of emesis once switched to granisetron and aprepitant after failure on ondansetron for RINV.
Acknowledgements
Funding: We thank the generous support of the Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Disclosure: The authors declare that there is no conflict of interest.
References
- Dennis K, Maranzano E, De Angelis C, et al. Radiotherapy-induced nausea and vomiting. Expert Rev Pharmacoecon Outcomes Res 2011;11:685-92. [PubMed]
- de Wit R, Aapro M, Blower PR. Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients? Cancer Chemother Pharmacol 2005;56:231-8. [PubMed]
- Van den Brande J, Brouwer A, Peeters M. Use of antiemetics in the prevention of chemotherapy-induced nausea and vomiting: review and focus on the Belgian situation. Acta Gastroenterol Belg 2014;77:240-8. [PubMed]
- Feyer P, Seegenschmiedt MH, Steingraeber M. Granisetron in the control of radiotherapy-induced nausea and vomiting: a comparison with other antiemetic therapies. Support Care Cancer 2005;13:671-8. [PubMed]
- Feyer PC, Maranzano E, Molassiotis A, et al. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 2011;19 Suppl 1:S5-14. [PubMed]
- Blower P, Aapro M. Granisetron vs ondansetron: is it a question of duration of 5-HT3 receptor blockade? Br J Cancer 2002;86:1662-3; author reply 1664.
- Wu CE, Liaw CC. Using aprepitant as secondary antiemetic prophylaxis for cancer patients with cisplatin-induced emesis. Support Care Cancer 2012;20:2357-61. [PubMed]
- Popovic M, Warr DG, Deangelis C, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 2014;22:1685-97. [PubMed]


