
Progenitor/stem cell transplantation for repair of myocardial infarction: Hype or hope?
Introduction
Although many novel avenues, such as cardiac resynchronization therapy (CRT), surgical cardiomyoplasty and artificial heart, have been taken into clinical practice in an attempt to optimize heart function after myocardial injury, a significant proportion of survivors will still develop congestive heart failure (CHF) and will suffer considerable functional limitation as a result. Pathologically, heart failure is the cause and consequence of myocardial remodeling in response to both ischemic or non-ischemic injury and a core component of this process is loss of cardiomyocytes (1). The central dogma for cardiac biology has been that the myocardium carries little capacity for self-regeneration. About ten years ago, the identification of progenitor/stem cells capable of contributing to myocardial regeneration has offered the hope that progenitor/stem-cell therapy could salvage the damaged heart.
In this review, we will discuss the current strategies and therapies targeting progenitor/stem cells in cardiac repair and regeneration, and present our ideas about the future of progenitor/stem cell based therapy in cardiovascular regenerative medicine.
Regenerative potential of the heart
Classical textbooks suggested that cardiomyocytes, like neurons, generally are believed to be terminally differentiated cells that do not proliferate after birth. But progress in recent years suggests we may have to look at this once again.
Hsieh PC et al. showed that progenitor/stem cells regenerate murine cardiomyocytes after heart injury, but are lost after one year of normal aging (2). Additionally, taking advantage of the incorporation of carbon-14 into DNA, Frisen et al. (3) found a way to determine the age of cardiomyocytes. They demonstrated that human cardiomyocytes renew themselves at an estimated rate of 1% per year at age 20, declining to 0.4% per year at age 75. At age 50, 55% of human cardiomyocytes remain from birth, while 45% were generated afterward. Although the turnover rate is low, the fact that it occurs at all makes it potentially appealing therapeutically. This discovery has raised another question: What is the heart response to injury? More recently, Porrello et al. proposed an answer. They found surgical resection of the ventricular apex in 1-day-old mice stimulates a regenerative response that appears to restore the damaged heart to its normal anatomy and function, although this was lost beyond 7 days of age (4). However, this study still has not answered whether the loss of this regenerative potential beyond 7 days is attributed to cell cycle arrest or to loss of mitogenic stimuli as the heart ages. Taken together, further studies should be exploited to examine the mechanism of the loss of the regenerative potential of the heart after birth. Hence stem/progenitor cells still hold the best hope for healing the injured heart.
What is the optimal cell type for regeneration of the failing heart?
In principle, transplantation of cells can replace damaged cardiomyocytes and restore heart function. But the ideal candidate cell types for transplantation should be satisfied the following items: (I) be immunocompatible within the donor heart; (II) integrate and synchronize with the rest of host myocardium.
Originally, the cells can be categorized into endogenous and exogenous cells. The many exogenous cell types include topipotent/pluripotent cells [embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) and adult cells of more limited potential (such as resident cardiac progenitor cells, circulating progenitor cells, and adult stem cells beyond the heart)]. Here we focus on the pluripotent cells relevant to clinical trials and those which are investigated most.
Bone-marrow-derived progenitor/stem cells
Hematopoietic progenitor/stem cells (HSC)
The tide of stem cell research in cardiovascular medicine was initiated by the report of Orlic D et al., which showed hematopoietic stem cells could transdifferentiate into cardiomyocytes when injected in the border zone of infarcted myocardium, making them of particular interest in the treatment of cardiac disease because they represent a well-characterized and ample source of progenitor cells.
These results, however, have not been confirmed by subsequent studies, which showed the hematopoietic stem cells do not transdifferentiate but instead become mature blood cells after transplantation (5,6), leaving the cardiac potential of HSC a controversial issue. Nevertheless, a number of landmark studies showed significant improvement in cardiac function when bone-marrow-derived cells were implanted directly or applied to the injured hearts by fabricated into cell sheet, implicating paracrine signaling as the major mechanism of action.
Therefore, the amelioration seen in ventricular function prompted a number of clinical trials using autologous bone marrow cells to treat the patients with heart failure/myocardial infarction. Early smaller clinical trials were exciting. However, larger, randomized, placebo-controlled and blinded studies have shown some mixed results (7-10). The REPAIR-AMI trial (the largest of the randomized, placebo-controlled trials) was positive in that it not only demonstrated improved left ventricular function, but also showed a reduction in the combined clinical endpoint of death, myocardial infarction or revascularization at one year (11). The BOOST trial also showed early improvement on left ventricular function (vs. control patients), but the difference had disappeared after 18 months. In contrast to the improved left ventricular function results of the REPAIR-AMI and BOOST trials, a double-blind, randomized controlled study, using autologous bone marrow MNCs in patients with myocardial infarction 24 h after successful percutaneous coronary intervention, showed no benefit in left ventricular ejection fraction, but a significant reduction in infarct size and improved regional left ventricular function (12). It should be emphasized that >99.9% of bone marrow mononuclear cells are not stem cells, but are committed, although immature, granulocytes or other hematopoietic lineages. These trials indicate that the delivery of bone marrow derivatives through the coronaries is feasible and safe, but the benefits are modest. Later, a meta-analysis of 18 randomized and nonrandomized trials involving 999 patients with acute myocardial infarction or chronic ischemic cardiomyopathy found that transplantation of adult bone marrow cells improved left ventricular ejection fraction by 5.40%, decreased infarct scar size by 5.49% and lowered left ventricular end-systolic volume by 4.80 mL (13). It has been challenging to draw any major conclusions thus far, in part because of size (only 2 trials have studied more than 100 patients) and in part because of the heterogeneity in trial design, type of cell under study, methods for cell preparation and storage, techniques for cell delivery, and timing of cell delivery. Regarding cell type, most clinical studies have used unfractionated bone marrow cells as the delivery product, postulating that stem and progenitor cells within this population are the biologically relevant therapeutic agents. Definitions of the relevant cell in this regard have in general been confined to historically defined hematopoietic progenitor cells, which are those that express the surface antigens CD34 and CD133. Given that the content of CD133 and/or CD34 cells has been only 0.1% to 5% of cells in most studies, and given that myocardial regeneration is almost certainly absent or minimal, it is difficult to conclude that any therapeutic benefit is caused by these progenitor cells alone.
Marrow-derived mesenchymal stem cells (MSCs)
MSCs, also referred to as connective tissue progenitor cells or multipotent mesenchymal stromal cells, are a common source of adult stem cells. They can be isolated from bone marrow stroma and adipose tissue, as well as in other organs (14) and are easy to be collected. Generally, they are defined based on their antigenic profile, that is, positive for stromal markers (CD44, CD49d, CD90, and CD166), negative for hematopoietic markers (CD14, CD45, and CD34), and negative for endothelial markers (VEGFR2, CD34, and CD105) and behavior in culture [i.e., capable of differentiation along several mesodermal cell lines (bone, nerve, fat, muscle, etc)]. For many reasons, MSCs are potentially a more attractive option for cell delivery as a therapy for left ventricular dysfunction. Perhaps of greatest importance with respect to their potential in cell therapy, MSCs are believed to be immune privileged, which promotes their use in allogeneic recipients (15,16).
Similar to HSC, human MSCs were originally reported to transdifferentiate into cardiomyocytes in the adult murine heart (17) but are now thought to exert their main actions through paracrine behavior (18) (Figure 1) .
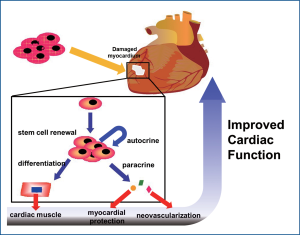
Previous experiment using media from MSC cultures exert therapeutic effects similar to those of direct MSC therapy, suggesting that effects via released cytokines may play a major therapeutic role in MSC therapy. Also, another group demonstrated that, in a model of murine hind limb ischemia, MSC transplantation enhanced tissue repair via secretion of multiple cytokines, including vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and placental growth factor (PlGF), rather than incorporation of MSCs into new or remodeling tissues. This beneficial effect is enhanced further when MSCs are genetically modified to express the pro-survival kinase Akt1 (19-21). Akt1-expressing MSCs secrete sFRP2 that makes cardiomyocytes more tolerant to hypoxia-induced apoptosis and improve myocardial function (18,21,22). The positive effects of transplanted MSCs, and the ability to genetically modify them, promote their use as a cellular vehicle to deliver angiogenic proteins [e.g., angiopoietin-1 (Ang1)] (23), survival factors [insulin-like growth factor-1 (IGF-1)] (24), chemokines [stromal cell-derived factor-1 (SDF-1)] (25) or Wnt antagonists (sFRP2) (26) to further enhance the recovery of damaged myocardium. Compared to direct intramuscular injection of MSCs into the peri-ischemic region, Miyahara et al. (27) transplanted monolayers of adipose tissue-derived mesenchymal stem cells into infarcted rat hearts, which resulted in improved fractional shortening and infarct wall thickness. After 4 weeks, the monolayers had expanded in situ to produce 600 µm-thick tissue where it was transplanted over the infarct scar. The newly formed tissue consisted of neovasculature, undifferentiated mesenchymal stem cells, and a few cardiomyocytes. They further observed a paracrine angiogenic effect, which altogether led to amelioration of heart function.
A recent report has shown that the administration of MSCs to pig infarcts stimulated resident cardiac stem cells to contribute to the repair of the infarcts (28). Further elucidation of MSC secreted paracrine mediators may provide a cell-free based paradigm for myocardial protection. For the clinical trials using MSCs, there are few published results with these cells, but one of the strongest cardiac-repair treatment effects seen so far indicates a 14% improvement in ejection fraction was reported after the intracoronary administration of large numbers of autologous MSCs (29). Allogeneic administration of MSCs into patients intravenously within ten days of infarction showed well tolerated, decreased arrhythmias and ameliorated contractile dysfunction (30).
Taken together, compelling evidence indicates that bone marrow cells do not work by directly differentiating into new cardiomyocytes, but elaborate signals that control the response of cells native to the myocardium, and thereby regulate healing.
Another issue should be addressed is the homing of MSC. Unlike traditional small molecule and biologic therapeutics, which upon initial infusion circulate throughout the body within the plasma compartment, MSCs tend to reproducibly aggregate in specific tissues. Recent studies have shown that, 48 hours after intravenous infusion in rats, more than 90% of infused cells can be detected in the lung (31), with virtually no cells detected in some potential target tissues, such as the brain (32). The degree to which MSCs demonstrate endogenous ‘homing’ has recently been called into question because of wide variation in experimental methodologies used to quantitate cells in tissue (33). Recent studies have elucidated several endogenous mechanisms that might be utilized to overcome this intrinsic bias in MSC biodistribution. The potential role of stromal cell-derived factor alpha (SDF-1) and its receptor, chemokine receptor 4 (CXCR4) in recruiting bone-marrow-derived cells to sites of injury was first described in models of vascular (34) and myocardial (35) injury. After acute ischemic myocardial injury, cardiac SDF-1 levels increase dramatically (36) and appear to serve as a homing signal for endogenous cells expressing CXCR4. One proposed therapeutic strategy has been to augment and temporally extend the expression of SDF-1α in order to increase the numbers of cells recruited from the circulation; this strategy is based on the hypothesis that SDF-1α homing signal may be rate limiting for recovery (37), and has shown promise in preclinical models of myocardial infarction (38). These defining studies have been expanded with an aim of increasing the homing and/or function of exogenously administered MSCs. Transplantation of MSCs overexpressing SDF-1α into acutely infarcted rodent hearts demonstrated incremental improvements in cardiac function when compared to control MSCs (39); however the exact mechanism for this effect remains unclear, as SDF-1α may act on multiple target cells. In a more direct approach, overexpression of CXCR4 in MSCs leads to an increase in vitro migration (40) as well as engraftment, ultimately resulting in improved overall cardiac function in the damaged heart after intravenous injection (41,42). In this case, the mechanism appears to be upregulation of matrix metalloproteinases (MMPs), which have recently been shown to be critical for MSC migration and tissue retention (43-45) and might prove to be useful tools to enhance MSC homing.
Endothelial progenitor cells (EPC)
EPC can be isolated from peripheral blood mononuclear cells and bone marrow, and have the potential to differentiate into endothelial cells. They can also differentiate into cardiomyocytes when co-cultured with neonatal rat cardiomyocytes (45). Injection of EPC into a heart with myocardial infarction was shown to improve cardiac function by promoting angiogenesis, without their differentiation into cardiomyocytes (46,47). Subsequently, the search began to find ways to enhance their mobilization or to directly incorporate them into the vasculature of injured tissues. Both VEGF and granulocyte colony-stimulating factor (G-CSF) have been shown to increase EPC mobilization from bone marrow. And statins (3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors) have been shown to stimulate the mobilization of EPCs from the bone marrow as well, pointing to yet another aspect of the ever-evolving understanding of the many therapeutic benefits of the drug (48).
This cell population arises from the hematopoietic lineage and possesses certain stem cell markers during the maturation pathway, such as CD34 in human (49). In recent years, researchers have suggested an easy process for isolation of these cells both in vitro and in vivo (49). Furthermore, the incorporation of EPCs in ischemic tissue has been shown to contribute to the recovery of ischemia through participation in neovascularization, both in animal study and in promising early clinical trials and their long term outcome (50-53). Masaaki et al. (54) have suggested a different mechanism by which EPCs protect cardiomyocytes in the scar area. These authors found that a rapid recruitment of EPCs to the myocardium occurs in response to very short periods of ischemia and that these cells express an array of potentially cardioprotective cytokines, including nitric oxide synthase isoforms.
In the TOPCARE-AMI trial, patients with myocardial infarction received 3-day-old EPCs in the infarct-related artery 4 days after infarction. Left ventricular function improved to a greater extent and infarct size was smaller after EPC infusion, compared with a historical control group and almost 10-year follow-up shows it long term safety and efficacy (50-53). While a double-blind prospective study is required to confirm these findings, the important observation was that EPC transfer appeared safe, as no malignant arrhythmias, inflammatory reactions or obstruction of blood vessels were observed. However, in another uncontrolled study, infusion of a subpopulation of CD133+ bone marrow cells in the infarct-related artery was associated with enhanced recurrent ischemia and need for repeat intervention of the infarct-related artery (55). Although this was unexpected and underlying causes remain unclear, these results highlight the need for continued awareness when administering in vitro purified cell populations. In patients with chronic coronary occlusion, recanalization and stent implantation was followed 10 days later by infusion of 4-day-old EPCs or placebo into the stented artery. Compared with placebo injection, EPC transfer resulted in a reduction of hibernating cardiac tissue, enhanced perfusion and an improvement in global cardiac function. This double-blind, randomized, placebo-controlled study thus suggests that cultured EPCs are able to improve cardiac function, even when administered more than 30 days after coronary occlusion (56). Whether the observed benefit was caused by EPC-mediated vasculogenesis or by an indirect paracrine effect on metabolism and hibernating myocardium remains unknown. A second randomized, controlled study compared the efficacy of transcoronary delivery of bone marrow mononuclear cells or 3-day-old EPCs to treat patients 3 months after myocardial infarction (57). In contrast to the previous study, recanalization of the infarct-related artery was performed immediately following the acute event. Compared with control patients without cell therapy, only mixed mononuclear cells, but not EPCs, were effective in enhancing left ventricular function. These data suggest that EPCs are not very effective when administered a long time after myocardial infarction. Mechanisms remain speculative, but it is possible that deficient EPC homing signals contribute to a low EPC incorporation and lack of a therapeutic effect.
Skeletal myoblast
Skeletal myoblasts, or satellite cells, are found in the basal membrane of muscle fibers and maintain the homeostasis of skeletal tissue (58,59). Myoblasts are easy to isolate from small muscle biopsies as they can proliferate and expand substantially in culture. Obvious similarities between skeletal and cardiac muscle tissue suggest that satellite cells may adopt a cardiomyogenic fate once inside ventricular tissue. Moreover, they are resistant to hypoxia-induced apoptosis, providing another potential advantage for them in repopulating the ischemic myocardium (60). Animal studies show that myoblasts that are injected into cardiac tissue after ischemic injury cause global and regional functional improvement (61-63). In a chronic heart failure dog model, transplantation of myoblasts also led to a significant recovery in left ventricular hemodynamics (64). In a similar fashion, magnetic resonance imaging showed that intramyocardial delivery of myoblasts in rabbits with an acute myocardial infarction demonstrated an increase in regional left ventricular wall thickness and a decrease in the deleterious effects of post-infarction cardiac remodeling (65). Despite the ability to incorporate into the infarct site and develop contraction-like characteristics, myoblasts generally fail to form intercalated disks and appropriate gap junctions with resident cardiomyocytes (66). The lack of electromechanical coupling with the surrounding host cardiac tissue also caused arrhythmias in a number of cases (67). Although straightforward application of skeletal myoblasts may have limited future use in cardiovascular cell therapy, satellite cells could offer an ample source of cellular material that is similar to cardiac progenitor cells and that might be reprogrammed with cardiac-specific regulatory factors. Skeletal myoblasts may also be engineered to form connections with resident cardiomyocytes by expressing appropriate gap junction proteins before transplantation (68). In the future, it should be addressed that whether induced pluripotent stem cells (iPSC) from skeletal myoblast (69) carry a better cardiomyogenic potential compared to other cell types regarding to the epigenetic memory in skeletal myoblast.
Cardiac progenitor cells (CPC)
Recent evidence suggests that a pool of CPCs resides in the adult myocardium (70). The discovery of various cardiovascular progenitor cells (CVPC) in adult hearts has provided exciting candidates that could replace the injured or aged cardiomyocytes. In some cases, these cardiovascular progenitor cells can improve cardiac function after implantation into injured hearts in animal models. However, the developmental origins and long-term benefits of such cardiovascular progenitor cells remain largely unknown or unproven. Yibing Qyang et al. recently isolated and characterized a novel population of cardiovascular progenitor cells from rodent and human hearts, as well as from murine ESCs (71-74). These cells are capable of making nearly an entire heart during embryonic and postnatal heart formation and are marked by expression of ISL1 - a LIM-homeo domain transcription factor. ISL1+ cardiovascular progenitor cells are highly proliferative, multi-potent cells that have the capacity to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells, all of which are required for cardiac repair.
An early primal ISL1 human heart progenitor cell has been isolated by using lineage tracing technology from human ESC, which gives rise to a well-characterized family of down-stream multipotent second heart field (SHF) heart progenitor cells that then generate diverse lineages (74). Few ISL1+ cells can be found in the heart at later embryonic stages, including the developing outflow tract. Moreover, ISL1+ cells generally coexpress with Flk-1 and Nkx2.5, indicating a rapid fate restriction. ISL1-expressing cells that do not express other markers such as FLK-1 or NKX2.5 are found in the developing heart including the outflow tract, which may represent the upstream precursor for the family of multipotent progenitors in the SHF lineage. But these primordial stem cells are found only in fetuses and could not be used for cardiac repair because they could develop into undesired cell types which may lead to adverse effect. Therefore, researchers need to isolate "intermediate" cells that are already heading for a particular fate. In the meantime, the primordial cells could be used for disease modeling and drug screening.
Another kind of CPC is termed C-kit+ cells, which are the most extensively studied. In humans, they are expressed in mature circulating cells such as hematopoietic stem cells and mast cells, telocytes, the thymic epithelium, and cardiomyocytes during development (75). After isolation from rat and human hearts, C-kit+ cells have been reported to give rise to cardiomyocytes, smooth muscle cells and endothelial cells. Some studies indicate that, when transplanted, C-kit+ cells induce large-scale regeneration of myocardial infarcts and contribute to the formation of new myocardium and vessels. An ongoing clinical trial (ClinicalTrials.gov identifier NCT00474461) is testing the safety and efficacy of autologous C-kit+ cells as an adjunctive treatment for patients undergoing coronary bypass surgery (CABG).
Another CPC population in clinical trials is cardiosphere-forming cells. These cells are isolated on the basis of their migration out of cultured cardiac tissue and form spheroids in suspension cultures. They are composed of a mixture of cells(C-kit+ and others from the stromal-vascular compartment). In vitro and in vivo studies after transplantation have shown CPCs can differentiate into cardiomyocytes and improve heart function post myocardial infarction (76). Based on these data, a clinical trial (CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) was Launched (began in May 2009, completed in February 2012) using autologous CPCs (NCT00893360). In The Lancet, Raj Makkar and colleagues reported the result of CADUCEUS. This was a proof-of-principle study of intracoronary delivery of cardiosphere-derived cells for patients with recent myocardial infarction (within 1.5-3 months) and left ventricular dysfunction (ejection fraction 25-45%). Although scar size and viable myocardial mass did not alter in eight controls, patients in the cardiosphere-derived cell group who were studied with cardiac MRI (excluding three patients with implantable defibrillators and four pending 12 month data) had a reduction of 28% in average scar mass at 6 months and 42% at 12 months. Along with data from the SCIPIO trial (77), which used C-kit-positive cells, results from CADUCEUS show that a different population of cardiac progenitor cells derived from adult human heart (cardiosphere-derived cells) can reduce infarct scarring in patients with recent myocardial infarction. Different from the previous studies showing autologous transplantation of CPC is associated with little clinical evidence of cardiac regeneration; this trial will alert physicians-scientists to the potential of cardiac regeneration as a new paradigm for treatment after myocardial infarction (78).
Embryonic stem cells (ESCs)
As introduced above, a number of different somatic human tissues have been proposed as the source of stem cells/progenitors able to generate de novo cardiomyocytes. However, the cardiac differentiation potential of these adult stem cells is always poor due to their limited plasticity, which precludes their differentiation into functional cardiomyocytes, while only ESCs and the related reprogrammed cells have been shown to posses the capacity to stably differentiate into functional cardiomyocytes in vitro (79).
The successful generation of human ESCs (hESCs) in the late 1990s represents a milestone in the cell-based regeneration area (80). ESCs have nearly unlimited self-renewal and expansion capability and possess the unique property to differentiate into various somatic cell types, including cardiomyocytes, from three germ layers in vitro (81). Thus this stem cell type represents a fascinating source of committed cardiomyocytes of potential use in cardiac cell therapies (82,83). Using mouse ESC-derived cardiomyocytes (mES-CMs) (84), firstly demonstrated that cardiomyocytes generated from ESCs were capable to form stable intracardiac grafts in uninjured rodent hearts. Then a serious of subsequent works has further proved the beneficial effects on heart function after transplantation of mES-CMs to the experimental animal models of acute myocardial infarction (85-87).
Cardiomyocyte induction from hESCs has been found to be much more intractable, and their spontaneous cardiac differentiation efficiency is very low. Thus analysis of their in vivo regenerative potential is greatly hampered by the lack of sufficient amount of cardiomyocytes (79). Upon the development of several efficient and reproducible cardiac differentiation methods recently (88,89), the application potential of hESCs-derived cardiomyocytes (hES-CMs) in cardiac cell therapies has been explored. Pioneering transplantation studies of hES-CMs showed that when injected to uninjured hearts, the hES-CMs could survive and formed myocardial tissue (90,91). When hES-CMs were transplanted to the experimental animal models of acute myocardial infarction, they were found to engraft into the diseased hearts and provided beneficial effects on the heart function (88). In addition, Kehat et al. (90) showed that transplanted hES-CMs could substitute for pacemaker cells in a swine model with complete atrioventricular block and elicit and ectopic rhythm compatible with the animal’s survival. This was further confirmed by Li’s group (92) that hES-CMs are capable of actively pacing quiescent, recipient, ventricular cardiomyocytes in vitro and ventricular myocardium in vivo.
These successful attempts in applying cardiomyocytes generated from ESCs to repair the infarcted hearts are encouraging. However, several challenges, including generation of highly-purified cardiomyocytes to exclude the formation of teratoma after transplantation, establishing methods to produce cardiomyocytes with higher degree maturation in vitro and developing approaches to overcome immune rejection and other causes of graft cell death, remain to be solved in the future. To further ensure the safety of the ES-CMs, their electrophysiological consequences as well as functional integration with the recipient heart after transplantation needs to be detailed and carefully assessed. Thus far, most our knowledge is gained based on hES-CM transplantation experience in rodent models. Clearly, studies in larger models such as swine and primate are warranted in the future.
Induced Pluripotent Stem Cells (iPSC)
One of the most remarkable discoveries of the last few years is that adult somatic cells can be reprogrammed to a pluripotent stem cell state by expression of just a few key transcription factors. The pioneering study by Yamanaka and colleagues showing that overexpression of Oct3/4, Sox2, Klf4, and c-Myc could reprogram mouse fibroblasts to a pluripotent state virtually indistinguishable from mouse ESCs opened major new avenues of research (93). This epigenetic reprogramming was rapidly extrapolated to the human system using either the same combination of reprogramming factors (94) or a slightly different combination of transgenes (OCT4, NANOG, SOX2, LIN28) (95).
Given that some of the virally encoded genes are oncogenes that may be reactivated after transplantation, it is clear that protocols permitting reprogramming without the use of viruses are essential before iPSCs can become a clinical tool. Very recently, it has been shown that human iPSC derivation can be achieved with transposon (96,97), episomal (98), direct protein delivery systems (85,99) and microRNA (100-102).
Recently, it has been demonstrated that iPS cells have the potential to produce derivatives of all three germ layers and can also differentiate into various lineages in culture, including cardiovascular cells (103-106). Very recently, cardiomyocytes derived from disease-specific iPSC (long QT syndrome) was obtained (107).
With the rapid development of iPSC research, it appears likely that cellular reprogramming may provide important tools for translational scientists aiming at generating a specific cell type for cell therapy.
Direct reprogramming into cardiomyocytes
In addition to the iPSC technology, where fully differentiated cells are reprogrammed to the fully undifferentiated ESC-like state, and subsequent differentiation of such cells can give the desired cell type, one can envision more direct ways of reprogramming cells. In a pioneering experiment conducted more than 20 years ago, Harold Weintraub and colleagues showed that forced expression of the myogenic transcription factor MyoD in cultured fibroblasts caused such cells to adopt the myocyte fate (108). Thus, it seemed plausible that at least some cells in the body may have a more plastic developmental identity than traditionally thought. Recently, Douglas Melton and colleagues have taken this concept one step further. By first establishing the transcriptional code for generation of endocrine, insulin-producing beta cells in the pancreas during embryonic development (109) and thereafter transducing exocrine cells with viruses expressing these factors (110), they showed that cellular reprogramming can be achieved not only in the Petri dish but also in vivo.
Based on a similar strategy, Ieda and colleagues successfully reprogrammed cardiac fibroblasts into cardiomyocyte-like cells (referred to as iCMs) via the ectopic expression of Gata4, Mef2c, and Tbx5 (111). These investigators began with a selection of 14 key genes related to cardiac development, including transcription factors and chromatin remodeling factors, and expressed them in cardiac fibroblasts isolated from neonatal hearts obtained from aMHC-GFP transgenic mice. Seven days following transduction, 1.7% of cells expressed GFP. By serial reduction of the 14 factors, it was found that the optimal combination of Gata4, Mef2c, and Tbx5 resulted in GFP expression in 20% of the cells. Approximately 6% cells were positive for both GFP and TNNT2 (a marker of the sarcomere structure of cardiomyocytes). The iCM is similar to cardiomyocytes regarding to their morphology, cardiac markers expression, electrical-physical properties, and histone modifications on key cardiac markers genes. However, the induction rate is quite low (~1 out of 20 fibroblasts). Later, Ding Sheng et al. invented a totally different “short cut” to efficiently reprogram fibroblasts into cardiomyocytes. First, they partially reprogrammed fibroblast to an intermediate state between fibroblast and iPSC. In order to prevent the cells from reaching a pluripotent state during the time, an inhibitor of Janus kinase/signal transducer and activator of transcription (JAK/STAT) was added into the cell cultures. Cells were then induced directly into cardiomyocytes using tissue culture conditions designed for cardiac differentiation rather than iPSC generation. The technology produced induced cardiomyocytes with 30-40% efficiency by reprogramming fibroblasts to a relatively restricted mesodermal or cardiac-restricted progenitor stage, rather than ES-like stage. This makes subsequent differentiation and purification procedures considerably less challenging and minimizes the risk of transplanting unwanted and potentially dangerous contaminating cells. Taken together, direct in vivo reprogramming look to be very promising in the near future.
Optimized routes for progenitor/stem cell transplantation into the injured heart?
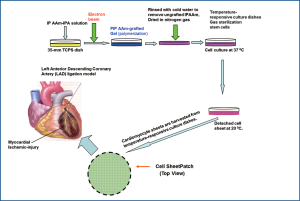
The routes of delivery must be improved in order to increase the efficacy of cardiac cell-based therapies. Suspension injection, the most commonly used often results in poor engraftment. Engineered heart tissues, generated by using biodegradable scaffolds, improved the efficiency of cell retention compared with cell suspension injection. However, this approach showed marginal benefit in improving cardiac function, possibly due to their limited attachment to the myocardium and the inflammatory and fibrotic responses caused by the scaffold’s degradation (112). A recently developed “cell sheet engineering” technology has greatly improved the efficiency and efficacy of cell engraftment. This system of delivery has resulted in the production of functional engineered heart tissue as well as a marked improvement in cardiac function following implantation (113,114). In order to prepare these cell sheets, tissue culture dishes are covalently coated with the temperature sensitive polymer, poly (N-isopropylacrylamide) (PIPAAm). At 37 ℃, the surface is hydrophobic and allows cells to adhere and proliferate. However, at 20 ℃, the surface becomes hydrophilic, leading to cell detachment because of rapid hydration and swelling of the grafted PIPAAm. The 3-D heart tissue can be established by layering these cell sheets. As there is no enzyme digestion involved, the cell surface, extracellular matrix (ECM), and cell-cell interactions remain intact in the detached cell sheet. Scientists have used this technology to develop engineered cell sheets with MSC (115), CPC (116) and induced adiopose (117). The intact adhesive molecules and ECM of the engineered tissues will enable to readily attach to the injured myocardium without any suture. Following implantation, these engineered tissues will engraft efficiently to the injured hearts, reducing scar formation and increasing cardiac function (Figure 2).
Perspectives
Recent progress has replenished our understanding of the development and stem cell biology of the cardiovascular system. On one hand, resident CPCs can be activated to enhance their migration from CPCs niches to the site of injury, proliferating to a crucial mass and differentiating into functional cardiomyocytes. On the other hand, extracardiac stem cells can be induced to potentiate their homing to the infarct heart and subsequent secretion of cardioprotective and resident CPCs -stimulating paracrine factors. Biotechnological advances such as genetic engineering and nanotechnology also provide further assistance in achieving this goal. Therefore, heart regeneration is not an imaginary hype but a realistic hope that deserves further advancement. However, some fundamental questions remain unanswered and need our further investigations. Why is there a need for so many different cell types participated in heart repair as a response to heart injury? Does exist a crucial event resulting from a damage mobilize all these soldiers for the sake of saving the dying heart? If stem cells are mobilized after injury, why does the function of fibrosis predominate? This is particularly disappointing, since the various stem cells that have been analyzed are able to produce cardiomyocytes, smooth muscle cells and endothelial cells in vitro. Is it because stem cell cannot survival in the harsh environment or they follow the road to fibrosis in order to survive in the hostile niche (inflammation, toxin etc.).
In recent years, we have acquired a lot of knowledge about the individual parts in the regenerative puzzle of the heart, but how to fit these together is still unclear. Our current challenge is to characterize the different stem cell-like populations in the heart and identify their respective roles during heart regeneration. It is also crucial to build a "roadmap" of how progenitor/stem cells are changed during a catastrophic injury such as a myocardial infarction. If progenitor/stem cells in the heart must choose between regeneration and scar formation, it is critical to know the mechanism/switch to operate the fate of the stem cells. This information will be beneficial to design novel strategies to promote the endogenous regenerative capacities of the adult heart and to optimize the cardiovascular differentiation of transplanted stem cells.
Acknowledgements
This work was supported by NIH grants, HL089824, HL081859, HL110740 (Yigang Wang) and National Natural Science Foundation of China, 31030050 (Huangtian Yang).
Footnote
No potential conflict of interest.
References
- Alcon A, Cagavi Bozkulak E, et al. Regenerating functional heart tissue for myocardial repair. Cell Mol Life Sci 2012; [Epub ahead of print].
- Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 2007;13:970-4.
- Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98-102.
- Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078-80.
- Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004;428:664-8.
- Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004;428:668-73.
- Assmus B, Schächinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17.
- Lunde K, Solheim S, Aakhus S, et al. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J 2005;39:150-8.
- Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210-21.
- Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 2006;367:113-21.
- Schächinger V, Erbs S, Elsässer A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 2006;27:2775-83.
- Wollert KC, Meyer GP, Lotz J,et al.Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial.Lancet2004364:141-8.
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989-97.
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726-36.
- Rodriguez AM, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med 2005;201:1397-405.
- Saito T, Kuang JQ, Bittira B, et al. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg 2002;74:19-24; discussion 24.
- Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93-8.
- Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A 2007;104:1643-8.
- Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 2006;14:840-50.
- Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195-201.
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367-8.
- Kung HJ. Targeting tyrosine kinases and autophagy in prostate cancer. Horm Cancer 2011;2:38-46.
- Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun 2007;357:779-84.
- Haider HKh, Jiang S, Idris NM, et al. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 2008;103:1300-8.
- Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197-207.
- Alfaro MP, Pagni M, Vincent A, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A 2008;105:18366-71.
- Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 2006;12:459-65.
- Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 2010;107:913-22.
- Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004;94:92-5.
- Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277-86.
- Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009;18:683-92.
- Harting MT, Jimenez F, Xue H, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 2009;110:1189-97.
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206-16.
- Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322-8.
- Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003;362:697-703.
- Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res 2009;104:1133-5.
- Ziegler M, Elvers M, Baumer Y, et al. The Bispecific SDF1-GPVI Fusion Protein Preserves Myocardial Function After Transient Ischemia in Mice. Circulation 2012;125:685-96.
- Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197-207.
- Zhao T, Zhang D, Millard RW, et al. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol 2009;296:H976-86.
- Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol 2008;44:281-92.
- Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med 2006;7:19-24.
- Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 2008;16:571-9.
- Steingen C, Brenig F, Baumgartner L, et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol 2008;44:1072-84.
- Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007;109:4055-63.
- De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007;92:440-9.
- Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis 2007;49:421-9.
- Narmoneva DA, Vukmirovic R, Davis ME, et al. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 2004;110:962-8.
- Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 2000;6:1004-10.
- Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001;103:2776-9.
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7.
- Leistner DM, Fischer-Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol 2011;100:925-34.
- Assmus B, Schächinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17.
- Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol 2004;44:1690-9.
- Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via "imported" nitric oxide synthase activity. Circulation 2005;111:1114-20.
- Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 2005;112:I178-83.
- Erbs S, Linke A, Adams V, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circ Res 2005;97:756-62.
- Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006;355:1222-32.
- Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev 2006;20:1692-708.
- Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev 2008;18:330-6.
- Menasché P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis 2007;50:7-17.
- Murry CE, Wiseman RW, Schwartz SM, et al. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest 1996;98:2512-23.
- Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 1998;4:929-33.
- Ghostine S, Carrion C, Souza LC, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 2002;106:I131-6.
- He KL, Yi GH, Sherman W, et al. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant 2005;24:1940-9.
- van den Bos EJ, Thompson RB, Wagner A, et al. Functional assessment of myoblast transplantation for cardiac repair with magnetic resonance imaging. Eur J Heart Fail 2005;7:435-43.
- Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol 2002;34:241-9.
- Leobon B, Garcin I, Menasche P, et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A 2003;100:7808-11.
- Roell W, Lewalter T, Sasse P, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 2007;450:819-24.
- Ahmed RP, Haider HK, Buccini S, et al. Reprogramming of skeletal myoblasts for induction of pluripotency for tumor-free cardiomyogenesis in the infarcted heart. Circ Res 2011;109:60-70.
- Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell 2008;132:537-43.
- Qyang Y, Martin-Puig S, Chiravuri M, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell 2007;1:165-79.
- Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006;127:1151-65.
- Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 2003;5:877-89.
- Bu L, Jiang X, Martin-Puig S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 2009;460:113-7.
- Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A 2009;106:1808-13.
- Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 2010;106:971-80.
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57.
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012;379:895-904.
- Rajala K, Pekkanen-Mattila M, Aalto-Setälä K. Cardiac Differentiation of Pluripotent Stem Cells. Stem Cells Int. 2011;383709.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7.
- Boheler KR, Czyz J, Tweedie D, et al. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002;91:189-201.
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol 2005;23:845-56.
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008;132:661-80.
- Klug MG, Soonpaa MH, Koh GY, et al. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 1996;98:216-24.
- Min JY, Yang Y, Converso KL, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 2002;92:288-96.
- Naito H, Nishizaki K, Yoshikawa M, et al. Xenogeneic embryonic stem cell-derived cardiomyocyte transplantation. Transplant Proc 2004;36:2507-8.
- Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med 2006;203:2315-27.
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015-24.
- Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008;453:524-8.
- Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004;22:1282-9.
- Laflamme MA, Gold J, Xu C, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol 2005;167:663-71.
- Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation 2005;111:11-20.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72.
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917-20.
- Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 2009;458:771-5.
- Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009;458:766-70.
- Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797-801.
- Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009;4:472-6.
- Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011;8:633-8.
- Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 2011;8:376-88.
- Li Z, Yang CS, Nakashima K, et al. Small RNA-mediated regulation of iPS cell generation. EMBO J 2011;30:823-34.
- Schenke-Layland K, Rhodes KE, Angelis E, et al. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 2008;26:1537-46.
- Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation 2008;118:498-506.
- Mauritz C, Schwanke K, Reppel M, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008;118:507-17.
- Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 2009;104:e30-41.
- Malan D, Friedrichs S, Fleischmann BK, et al. Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circ Res 2011;109:841-7.
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987;51:987-1000.
- Zhou Q, Law AC, Rajagopal J, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 2007;13:103-14.
- Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627-32.
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86.
- Li RK, Jia ZQ, Weisel RD, et al. Survival and function of bioengineered cardiac grafts. Circulation 1999;100:II63-9.
- Masuda S, Shimizu T, Yamato M, et al. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev 2008;60:277-85.
- Miyagawa S, Sawa Y, Sakakida S, et al. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation 2005;80:1586-95.
- Zhang D, Huang W, Dai B, et al. Genetically manipulated progenitor cell sheet with diprotin A improves myocardial function and repair of infarcted hearts. Am J Physiol Heart Circ Physiol 2010;299:H1339-47.
- Zakharova L, Mastroeni D, Mutlu N, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res 2010;87:40-9.
- Imanishi Y, Miyagawa S, Maeda N, et al. Induced adipocyte cell-sheet ameliorates cardiac dysfunction in a mouse myocardial infarction model: a novel drug delivery system for heart failure. Circulation 2011;124:S10-7.


