Gold nanorods for target selective SPECT/CT imaging and photothermal therapy in vivo
Abstract
The development of theranostic agents with high detection sensitivity and antitumor efficacy at low concentration is a challenging task for target selective imaging and therapy of cancers. In this study, folate-conjugated and radioactive-iodinelabeled gold nanorods (GNRs) were designed and synthesized for target selective SPECT/CT imaging and subsequent thermal ablation of folate-receptor-overexpressing cancers. Both (ortho-pyridyl) disulfide-poly(ethylene glycol)-folate and a short peptide, H2N-Tyr-Asn-Asn-Leu-Ala-Cys-OH, were conjugated on the surface of the GNRs through thiol chemistry. The tyrosine in the peptide sequence was introduced for radioactive-iodine labeling through an iodine-tyrosine interaction. The labeling efficiency of radioactive iodine was more than 99%. Radiochemical stability tests on iodine-125-labeled GNRs in human serum showed that 91% of the iodine-125 remained intact on the GNRs after incubation for 24 h. In vitro and in vivo results in this study confirmed the potential utility of folate-conjugated and iodine-125-labeled GNRs as smart theranostic agents. This type of platform may also be useful for the targeted SPECT/CT imaging and photothermal therapy of inflammatory diseases such as atherosclerosis and arthritis, in which folate-receptor-overexpressing macrophages play pivotal roles.
Keywords
Target selective imaging; gold nanorods; SPECT/CT; photothermal therapy
Introduction
Theranostic agents for simultaneous cancer diagnosis and therapy have received a great deal of attention. In particular, a variety of nanomedicines have been designed as theranostics due to their advantageous properties, which include a relatively large surface area for tagging targeting ligands and contrast agents, enhanced permeation and retention (EPR) in the circulatory system, and size- and shape-dependant optical and magnetic properties (1-6).
Receptor molecules overexpressed on the surface of cancer cells have been used as important diagnostic and therapeutic targets for the detection and therapy of human cancers. However, there are a limited number of target receptors on the cancer cell surface for specific binding with targeting ligands. Therefore, development of theranostic agents with high detection sensitivity and antitumor efficacy at low concentration is desirable for the target selective imaging and therapy of cancers. Intriguingly, the detection sensitivity of single-photon emission computed tomography (SPECT) imaging is relatively high (i.e., in the picomolar range), which enables the visualization of whole-body biological processes in vivo without inducing pharmacological effects (7-9). Thus this technique has been widely used for the molecular imaging of various diseases after labeling probes with radioisotopes (for example, radioactive iodine isotopes and technetium-99m). In addition to SPECT imaging, which provides molecular information with no depth limitation, additional anatomic information using x-ray computed tomography (CT) is very helpful for accurate interpretation of the images obtained. Therefore, SPECT/CT fusion imaging is generally used in clinical applications to obtain an improved imaging contrast and anatomic information (10,11).
Recently, photothermal therapy using biocompatible plasmonic nanomaterials has received considerable attention as a new candidate technique for cancer treatment. Among several types of plasmonic metal nanoparticles, including nanoprisms, nanoshells, and spherical particles, gold nanorods (GNRs) have proved to be the most suitable for in vivo photothermal therapy because of their high light absorption coefficient in the near-infrared (NIR) region (600-900 nm) (4,12), excellent photothermal stability during laser illumination (13,14), and a better heat generation rate than other nanoparticles (15). It would be ideal if GNRs can be simultaneously used for enhanced CT imaging and the subsequent photothermal therapy of the cancers. However, our recent study showed that the optimum concentration of GNR for effective and homogeneous thermal ablation is less than 0.5 nM which is considerably smaller than that required for CT enhancement (13). On the basis of this observation, we speculate that labeling GNRs with radioisotopes and targeting ligands will prove to be a successful application of GNRs as a theranostic tool for target selective imaging and therapy.
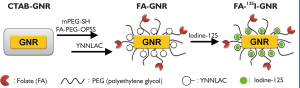
Folate receptor (FR) is one of the representative targets for cancer imaging or therapy because this receptor is overexpressed in various epithelial cancers, e.g., ovarian, brain, breast, and kidney (16). In particular, FR overexpression is observed in approximately 90% of ovarian carcinomas (17,18). Several FA-conjugated drugs for FR targeting are currently under assessment in human clinical trials (16,19). Here, we describe the application of folate-conjugated (FA-conjugated) and radioactive iodine-labeled GNRs for in vivo SPECT/CT imaging and subsequent photothermal therapy of folate receptoroverexpressing cancers (Scheme 1).
Experimental
Materials
Cetyltrimethylammonium bromide (CTAB, 99%), Gold (III) chloride trihydrate (HAuCl4, 99.9%), sodium borohydride (NaBH4, 99%), silver nitrate (AgNO3, 99%), folic acid (97%), dimethyl sulfoxide (DMSO, HPLC grade) and acetonitrile (ACN, HPLC grade) were obtained from Sigma-Aldrich (St. Louis, USA). RPMI 1640, heat-inactivated fetal bovine serum and Antibiotic & Antimycotic were purchased from Gibco® (Calsbad, USA). (ortho-pyridyl) disulfide-poly(ethylene glycol)-succinimidyl ester (OPSS-PEG-NHS, MW 5 kDa) was purchased from Creative PEGworks (Winston Salem, USA). Monomethoxy poly(ethylene glycol)-thiol (mPEG-SH, MW 5 kDa) was purchased from SunBio (Anyang, Republic of Korea). Peptide (YNNLAC, MW 696.29 Da) was purchased from Peptron Co., Ltd (Daejon, Republic of Korea). Iodogen (Pierce pre-coated iodination tube) and BCA assay kit were purchased Pierce Biotechnology (Rockford, USA). APCconjugated antihuman FOLR1 (folate receptor 1) antibody was purchased from R&D Systems, Inc. (Minneapolis, USA). [I- 131]NaI was purchased from Korea Atomic Energy Research Institute (KAERI) (Daejeon, South Korea). [I-125]NaI was purchased from PerkinElmer (Santa Clara, USA). Inrared camera (Thermovision A40) was obtained from FLIR Systems, Inc. (Wilsonville, USA). Ultrapure distilled water was used in all experiments.
Preparation of CTAB-coated GNRs
CTAB-coated GNRs were prepared by a seed-mediated method as described by Hongwei et al. (20). Briefly, CTAB solution (7.5 mL, 100 mM CTAB in deionized water) was mixed with 250 μL of 10 mM HAuCl4 aqueous solution. While the mixture was stirring at 400 rpm, 600 μL of 10 mM NaBH4 were added, stirred at 900 rpm for 2 min, and then maintained at 25 °C for 2 h, thereby preparing a seed solution. Meanwhile, 1.7 mL of a 10 mM HAuCl4 aqueous solution were added to 40 mL of a 100 mM CTAB aqueous solution, followed by sequential addition of 250 μL of a 10 mM AgNO3 aqueous solution and 270 μL of a 100 mM ascorbic acid aqueous solution. Then, 420 μL of the seed solution were added and reacted for 12 h. The product solution was centrifuged at 12000 rpm at 25 °C for 15 min, thereby obtaining the CTAB-coated GNRs.
Preparation of folate-conjugated GNRs, FA-GNR
Amine-functionalized folate, FA-miniPEG-NH2 , was synthesized as described by Dhar et al. (see Figure S1) (21).
An aqueous solution containing CTAB-coated GNRs was centrifuged at 12000 rpm for 15 min, decanted, and resuspended in deionized water to remove excess CTAB. The particle concentration of CTAB-coated GNRs was 50 nM after resuspension. FA-miniPEG-NH2 (1.25 μmol) was reacted with OPSS-PEG-NHS (357 nmol) in PBS (6.7 mM, pH 7.4, NaCl 154 mM) for 2.5 h at 25 °C to prepare FA-PEG-OPSS. Deionized water (500 μL) containing both FA-PEG-OPSS (75 nmol) and mPEG-SH (175 nmol) were added to 500 μL of CTAB-coated GNRs at 25 °C for 1 h to couple both PEGs on the GNR surface, thereby obtaining FA-coupled GNRs. After 1 h, the mixture was centrifuged at 12000 rpm for 15 min, decanted, and resuspended in 500 μL of PBS to remove unconjugated FA-PEG-OPSS and mPEG-SH. Then, a tyrosine-containing short peptide, YNNLAC, was further conjugated on the surface of the FA-coupled GNRs. The YNNLAC peptide was dissolved in PBS to prepare a 2.5 mM YNNLAC peptide solution. Then, the YNNLAC solution (100 μL) was added to 500 μL of FA-coupled GNR solution and stirred at 25 °C for 12 h. To remove unconjugated peptides, the mixture was centrifuged at 12000 rpm for 15 min, decanted, and resuspended in 500 μL of PBS, thereby finally obtaining FAGNR in which both the FA-PEG and YNNLAC peptides were conjugated on the surface of the GNRs.
The number of conjugated YNNLAC per GNR was calculated by analyzing the amount of unconjugated peptides in the supernatant by using high-performance liquid chromatography (HPLC). HPLC analysis was performed on a Waters 2690 (Waters, USA) HPLC system equipped with XTerra RP18, 5 μm, 4.6×250 mm reverse-phase column. The mobile phase for HPLC analyses was acetonitrile (0.1% trifluoroacetic acid) with a flow rate of 1 mL/min at room temperature. The concentration of the GNRs was calculated using the extinction coefficients (1.3×109 and 4.6×109 M−1 cm–1 at 510 and 785 nm, respectively) (12). The absorption spectrum of the GNR solution was measured using a UV/Vis scanning spectrophotometer (DU730, Beckman Coulter, Brea, CA, USA). TEM experiments were performed using a transmission electron microscope (TEM; JEM-1010, JEOL, Tokyo, Japan), operating at 40–100 kV.
Dispersion stability of FA-GNR
To check the dispersion stability of FA-GNR in the various aqueous solutions, CTAB-coated GNR and FA-GNR were dispersed in each of deionized water, PBS, and a cell culture medium (RPMI 1640 without phenol red) containing 10% fetal bovine serum (FBS). The final concentration of GNRs in the solutions was adjusted to 1 nM. The solutions were maintained at 25 °C and observed for 7 d. White-light images and UV/Vis spectra of the sample solutions were taken periodically.
In vitro cell studies
In vitro cell uptake and phototoxicity tests were performed to evaluate the ability of FA-GNR to selectively target FR-positive ovarian cancers. A human ovarian carcinoma (SKOV3) cell line and human lung adenocarcinoma epithelial cell line (A549) were obtained from the American Type Culture Collection (Rockville, MD, USA), and were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% Antibiotic & Antimycotic in a humidified 5% CO2 incubator at 37 °C. In this study, both cell lines were adapted to grow in folate-deficient RPMI medium for 1 week.
Flow cytometric analysis of folate receptor (FR) expression
The flow cytometric analysis of folate-receptor expression in the cell lines was carried out using an APC-conjugated antihuman FOLR1 (folate receptor 1) antibody according to the manufacturer’s instructions. SKOV3 and A549 cells were trypsinized and then centrifuged at 1000 rpm for 3 min. The cells were washed with PBS and resuspended in 1 mL of PBS at a concentration of 2×105 cells/mL. 10 μL of APC-FOLR1 were added and incubated in the dark for 15 min. The cells were washed with PBS to remove unbound antibodies. The cells were resuspended in PBS for the final flow cytometric analysis.
In vitro cell uptake test
Labeling of tyrosine (Y) with the radioisotope I-131 was performed for quantitative analysis of FR-mediated cell uptake. FA-GNR (39 μL, 43.8 nM particle concentration) and 103.5 MBq of [131I]NaI in 0.2 mL of saline were added to an Iodogen (Pierce pre-coated iodination tube) and then incubated at 25 °C for 10 min. Radio iodination was checked by ITLC (0.9% saline). Since the labeling efficiency was more than 99%, no further purification was performed to obtain the 131I-labeled FAGNRs (i.e., FA-131I-GNR).
In brief, SKOV3 and A549 cells were both seeded in triplicate in each well of 24-well plates at a density of 3×104 cells/well and incubated for 24 h for cell attachment. The growth medium was removed from the cell cultures and replaced with 200 μL of FAfree culture medium containing 37 kBq/mL of FA-131I-GNR. In free-FA competition studies, the cells were pre-incubated with 1 mM free-FA-containing culture medium at 37 °C for 30 min and then co-incubated with FA-131I-GNR for an additional 4 h. The cells were then quickly washed twice with 500 μL of icecold Dulbecco’s phosphate-buffered saline (DPBS, Welgene) to remove the unbound GNRs. Cellular radioactivity was released with 200 μL of lysis buffer (sterile water containing 0.1% SDS) and counted in a gamma-counter. The protein content of each well was determined using a BCA assay kit. In vitro cell uptake was quantitated by measuring the radioactivity of cell extracts. The amount of protein per well was used for normalization of CPM results. The numerical values (CPM/μg) for the case and control group were represented by normalized fold ratios. Statistical significance was calculated by determining p values using Student’s t-test.
Cell viability test
SKOV3 cells were seeded in each well of 96-well plates at a density of 0.7×104 cells/well and incubated for 24 h for cell attachment. FA-GNR in aqueous solution was diluted with a cell culture medium containing 10% FBS to obtain various concentrations of FA-GNR (0.25, 0.5, 1, and 2 nM, based on GNR). The existing culture medium was replaced with 200 μL of fresh medium containing FA-GNR, and the cells were incubated for 4 h. For the untreated control group, the same volume of fresh culture medium, without GNRs, was added to the plate. After the cells were washed twice, a fresh cell culture medium was added, and further incubated for an additional 24 h. The cell viability of SKOV3 was analyzed using a CCK-8 solution. The absorbance was measured at 450 nm (reference =650 nm) using a microplate reader (Tecan Safire 2, Switzerland). Untreated control cells served as 100% viable cells, and the medium served as the background. Data are expressed as the mean (SD) from four data samples. The Student’s t-test was used for statistical analyses.
Selective photothermal cancer therapy using FA-GNR
SKOV3 cells were seeded in each well of 24-well plates at a density of 3×104 cells/well and incubated for 24 h for cell attachment. The existing culture medium was replaced with 200 μL of fresh medium containing FA-GNR (0, 0.25, 0.5, 1, and 2 nM, based on GNR), and the cells were incubated for 4 h. In free-FA competition studies, 1 mM FA was pre-incubated at 37 °C for 30 min and then coincubated with FA-GNR for further 4 h. After the cells were washed twice, a fresh cell culture medium was added. For the photothermal therapy studies, light was applied to the central part of the cell layer using an 810-nm CW laser (2 W/cm2 for 4 min, light-spot diameter: 3.5 mm). A calcein AM staining kit (Molecular Probes) was used as an indicator in live/dead viability and cytotoxicity tests. Following illumination, the cells were immediately stained with calcein AM for fluorescence microscopy. Live cells were stained with calcein (green 494/517 nm) and the nuclei of dead cells were stained with ethidium bromide dimer (red 528/617 nm). Fluorescence images were taken from more than three different areas in each case. All the data were acquired using identical settings on the microscope in order to ensure reproducibility.
In vivo animal studies
All animal studies were approved by the Institutional Animal Care and Use Committee. Female athymic nude mice (Balb/c-nu, ca. 19–21 g) were used for the in vivo experiments. SKOV3 cells (1×107 cells/50 μL of RPMI) were implanted subcutaneously into the left hind flank of each mouse, and the tumor size was measured daily (22). The animals were chosen for in vivo studies when their tumor sizes reached about 60 mm3.
Prepareation of 125I-labeled FA-GNR, FA-125I -GNR
Labeling of tyrosine (Y) with the radioisotope I-125 was performed for an in vivo SPECT imaging. FA-GNR (39 μL, 32.1 nM particle concentration) and 74 MBq of [125I]NaI in 0.1 mL of saline were added to an Iodogen (Pierce pre-coated iodination tube) and then incubated at 25 °C for 15 min. Radioiodination was checked by ITLC (0.9% saline). Since the labeling efficiency was more than 99%, no further purification was performed to obtain the 125I-labeled FA-GNRs (i.e., FA-125IGNR). Radiochemical stability tests were done by ITLC (saline) in human serum at 37 °C.
SPECT/CT imaging of ovarian cancer xenografts
For in vivo SPECT/CT fusion imaging, three mice received FA 125I-GNR at a dose of 40.7 MBq/mouse (equal to 0.841 pmol FA-GNR/mouse), and three other mice received FA-125I-GNR at a dose of 18.5 MBq/mouse (equal to 0.374 pmol FA-GNR/ mouse). SPECT images were obtained using animal SPECT (NanoSPECT, Bioscan) at 4 h and 24 h post-injection. CT images were obtained using animal PET/CT (Explore Vista- CT, GE) at 24 h post-injection. The obtained SPECT and CT scans were fused using InVivoScopeTM software (Bioscan). For comparison, SPECT images were also obtained from two mice without tumors, after intravenous injection of FA-125I-GNR at a dose of 18.5 MBq/mouse.
Tumor photothermal therapy
For in vivo tumor photothermal therapy, tumors in the FA-125IGNR- treated mice (3 mice, 0.841 pmol FA-GNR/mouse) were illuminated with an 810-nm CW laser (2 W/cm2, 600 J/cm2) for photothermal therapy at 24 h post-injection. For comparison, additional mice received intravenous injections of sterilized PBS solution (4 mice, 90 μL/mouse), followed by light illumination using an 810-nm CW laser (2 W/cm2, 600 J/cm2) 24 h after injection. Temperature increases in the tumor tissues during light illumination were monitored in real time using an infrared (IR) camera to obtain thermal images of both the PBS-treated and FA-125I-GNR-treated mice. The temperature increases were analyzed using ThermaCAMTM Researcher software. Thereafter, tumor volumes were measured periodically until day 30.
Results and Discussions
A total synthetic procedure for radioactive-iodine-labeled and FA-conjugated GNRs is illustrated in Scheme 1. Cetyltrimethylammonium bromide (CTAB)-coated GNR was synthesized by a seed-mediated method (20). Because of its cytotoxicity, excess CTAB was removed by centrifugation and redispersed in distilled water (DW). For the introduction of FAcoupled poly(ethylene glycol) (PEG) on the GNR surface, we synthesized an amine-functionalized folate, FA-miniPEG-NH2, and then coupled the FA-miniPEG-NH2 with (ortho-pyridyl) disulfide-poly(ethylene glycol)-succinimidyl ester (OPSS-PEGNHS, MW 5 kDa), obtaining an FA-coupled PEG, FA-PEGOPSS. Both FA-PEG-OPSS and monomethoxy-poly(ethylene glycol)-thiol (mPEG-SH, MW 5 kDa) were conjugated to CTAB-coated GNRs by a gold–thiol reaction. Then a short peptide, H2N-Tyr-Asn-Asn-Leu-Ala-Cys-OH (YNNLAC), was further conjugated on the surface of the GNRs through thiol chemistry, thereby finally obtaining FA-GNR in which both the FA-PEG and YNNLAC peptides were conjugated on the surface of the GNRs. The tyrosine (Y) in the peptide sequence is introduced for radioactive-iodine labeling through an iodine– tyrosine interaction. Hydrophobic amino acids leucine (L) and alanine (A) were introduced to promote self-assembly of the peptides on the GNR surface. From the high-performance liquid chromatography (HPLC) analysis, the molar ratio of conjugated YNNLAC per GNR was calculated at 6382:1.
The prepared FA-GNR was characterized by transmission electron microscopy (TEM) and UV/Vis absorption spectroscopy (Figure 1A). The average length and diameter of the GNRs were 32.9±3.5 nm and 9.58±1.5 nm, respectively. The absorption band of FA-GNR is unchanged after surface modification of the GNRs.
To check the dispersion stability, FA-GNR was dispersed in each of deionized water, PBS solution, and cell culture medium containing 10% fetal bovine serum (FBS). The white-light images and UV/Vis spectra of all the test solutions were taken after 10 min, 4 h, 24 h, and 168 h of incubation at 25 °C (Figure S2). The color and UV/Vis spectra of all the test solutions did not change for 168 h, as shown in Figure 1B. On the other hand, CTAB-coated GNRs rapidly formed aggregates in cell culture medium containing 10% FBS (23). This result indicates that PEGs on the surface of the FA-GNR were enough to prevent interaction between serum proteins and the GNRs, which is particularly important for achieving lengthy blood-circulation of the GNRs in vivo.
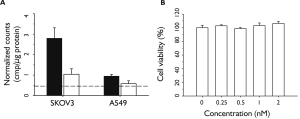
We evaluated the ability of FA-GNR to selectively target FRpositive ovarian cancers. A human ovarian carcinoma (SKOV3) cell line and human lung adenocarcinoma epithelial cell line (A549) were used as FR-positive and FR-negative controls, respectively. The expression levels of FR on both cell lines were confirmed by fluorescence-activated cell sorting (FACS) analysis after staining the cells with APC-conjugated antihuman FOLR1 (folate receptor 1) antibodies (Figure S3). For quantitative analysis of FR-mediated cancer targeting, a radioisotope, iodine-131, was used to label tyrosine residues in FA-GNR through iodine–tyrosine interactions to obtain 131I-labeled FA-GNR, FA-131I-GNR. Both SKOV3 and A549 cells were incubated with FA-131I-GNR for 4 h in the absence or presence of free FA (1 mM) as a competitor. The level of uptake was then calculated by measuring the radioactivity of cell lysates. The cellular uptake of FA-131I-GNR in SKOV3 cells was about 2.7-fold higher than in A549 cells (Figure 2A). When free FA (1 mM) was treated in SKOV3 cells and A549 cells along with FA-131IGNR, the cellular uptake of FA-131I-GNR decreased in both cell lines. In particular, an obvious decrease in the uptake of FA-131IGNR was observed in SKOV3 cells.
We next evaluated the in vitro cytotoxicity of FA-GNR in SKOV3 human ovarian carcinoma cells (Figure 2B). SKOV3 cells were incubated with FA-GNR solutions at various concentrations (0, 0.25, 0.5, 1, and 2 nM, based on GNR) and the viability of the cells was then checked. No cytotoxic effects of FA-GNR were observed at the tested concentrations.
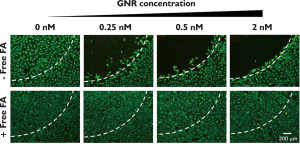
The in vitro photothermal therapy of FA-GNR was then evaluated in SKOV3 cancer cells. SKOV3 cells were incubated with various concentration of FA-GNR (0, 0.25, 0.5, and 2 nM based on GNR). After 4 h, the cells were washed and illuminated with an 810-nm CW laser (2 W/cm2, 4 min) and then stained with calcein AM. The cells which were treated with 0.25, 0.5, and 2 nM FA-GNR were shown to be dead in the light-illuminated region (Figure 3, upper row). However, cells co-incubated with 1 mM free FA as a competitor were alive upon laser illumination (Figure 3, bottom row). These results support the idea that FA-GNR was selectively internalized into SKOV3 cells by FRmediated endocytosis, and the subsequent photothermal effect of FA-GNR was adequate for cancer therapy in vitro.
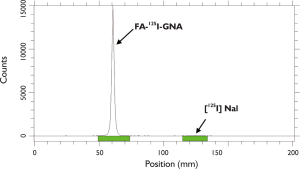
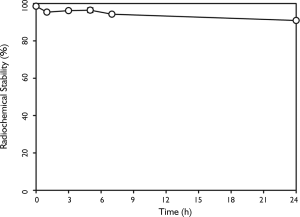
Prior to in vivo experiments, we used iodine-125 to label FA-GNR to obtain FA-125I-GNR for in vivo SPECT imaging. The labeling efficiency was more than 99% (Figure 4). Radiochemical stability of FA-125I-GNR in human serum showed that 91% of the 125I remained intact on the GNRs after 24 h of incubation at 37°C (Figure 5).
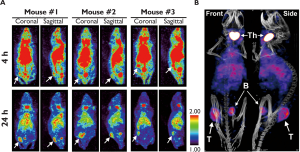
Three mice bearing subcutaneous SKOV3 tumors received intravenous injections of FA-125I-GNR when their tumor size reached about 60 mm3. The injection dose of FA-125I-GNR was 40.7 MBq/mouse, which equals 0.841 pmol FA-GNR/mouse. SPECT images were then obtained 4 h and 24 h after injection (Figure 6). CT images were also obtained 24 h after injection and then fused with SPECT images using InVivoScopeTM (Bioscan, USA) software. As shown in Figure 6, the tumor sites were starting to be discriminated from the surrounding tissues, and were clearly visualized, with good signal-to-noise ratio, at 24 h post-injection. Signals from the heart at 24 h post-injection indicated that FA-GNR was still circulating in the bloodstream. Signals from the thyroid and bladder resulted from accumulation of released 125I from the FA-125I-GNR during circulation. It is known that hepatic deiodases are capable of deiodonating mono- and di-iodotyrosine, resulting in the production of free radioiodide and eventually leading to thyroid uptake (24).
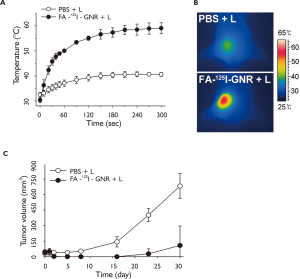
The in vivo therapeutic efficacy was further evaluated by measuring tumor growth rates. When their tumor sizes had reached approximately 60 mm3, the mice received an intravenous injection of either sterilized PBS solution (n=4) or FA-125I-GNR solution (n=3, 0.841 pmol/90 μL/mouse) (day0). Tumors in the PBS-treated and FA-125I-GNR-treated mice were illuminated with an 810-nm CW laser (2 W/cm2, 5 min) for photothermal therapy at 24 h post-injection at the same light dose and dose rate (day1). The temperature increase at the tumor sites was monitored in real-time during laser illumination using an IR camera. The tumor temperature in the FA-125I-GNR-treated group rose to 50 °C within 1 min, and thereafter gradually reached 59 °C at 5 min. The tumor temperature in the PBStreated group did not increase above 40 °C (Figure 7A and B). It is well known that the conditions for thermal ablation therapy are dependent on the operating temperature and time. It took 60 min to induce irreversible cellular damage at 46 °C, but 4–6 min is sufficient at 50–52 °C (25).
In vivo therapeutic effects were observed in the FA-GNRtreated groups compared with PBS-treated group after day 2 (Figure 7C). As expected, tumors in the FA-125I-GNR-treated group were shown to be mostly ablated the next day and disappeared after light illumination, whereas no antitumor effect was observed in the PBS-treated group. No apparent tumor mass was detected in any of the 3 mice in the FA-125I-GNR-treated group until day 15. On day 23, regrowth of the tumor mass was observed in 1 of the 3 mice. However, no tumor regrowth was detected in the other 2 mice until day 30. The mean tumor sizes in the FA-125I-GNR-treated group on day 30 was 15.9% (P=0.004) that in the PBS-treated group.
We also tested the dose effect of FA-125I-GNR on cancer imaging and photothermal therapy. Three additional mice with subcutaneous tumors received FA-125I-GNR at a dose of 18.5 MBq/mouse (equal to 0.374 pmol FA-GNR/mouse), and then SPECT images were obtained 4 h and 24 h post-injection. Light illumination at the tumor sites was performed at 24 h postinjection under the same conditions as described above. The tumor sites were clearly visualized from the SPECT images at 24 h post-injection (Figure S4). However, the temperature in the tumor tissues reached a plateau after 2 min and reached only 46 °C, even after 3 min of laser illumination, which is not enough to induce irreversible damage of tumors (Figure S5).





Conclusion
In conclusion, we showed in this study that FA-conjugated and radioactive-iodine-labeled GNRs are promising for SPECT/CT imaging and subsequent thermal ablation of FR-overexpressing ovarian cancer. This type of platform may also be useful for the targeted SPECT/CT imaging and photothermal therapy of inflammatory diseases such as atherosclerosis and arthritis, in which FR-overexpressing macrophages play pivotal roles (26-30).
Acknowledgements
This work was supported by a National Cancer Center grant from the Republic of Korea (1010150-2), and the Pioneer Research Center Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2011-0002110).
References
- Cole AJ, Yang VC, David AE. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol 2011;29:323-32.[LinkOut]
- Kelly KL, Coronado E, Zhao LL, et al. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 2003;107:668-77.[LinkOut]
- Murphy CJ, Sau TK, Gole AM, et al. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J Phys Chem B 2005;109:13857-70.[LinkOut]
- Jain PK, Huang X, El-Sayed IH, et al. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res 2008;41:1578-86.[LinkOut]
- Jun YW, Lee JH, Cheon J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew Chem Int Ed Engl 2008;47:5122-35.[LinkOut]
- Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 2008;60:1252-65.[LinkOut]
- Brandon D, Alazraki A, Halkar RK, et al. The role of single-photon emission computed tomography and SPECT/computed tomography in oncologic imaging. Semin Oncol 2011;38:87-108.[LinkOut]
- Chrastina A, Schnitzer JE. Iodine-125 radiolabeling of silver nanoparticles for in vivo SPECT imaging. Int J Nanomedicine 2010;5:653-9.[LinkOut]
- Lorberboym M, Minski I, Macadziob S, et al. Incremental diagnostic value of preoperative 99mTc-MIBI SPECT in patients with a parathyroid adenoma. J Nucl Med 2003;44:904-8.[LinkOut]
- Yamamoto Y, Nishiyama Y, Monden T, et al. Clinical usefulness of fusion of 131I SPECT and CT images in patients with differentiated thyroid carcinoma. J Nucl Med 2003;44:1905-10.[LinkOut]
- Chen L, Luo Q, Shen Y, et al. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med 2008;49:1952-7.[LinkOut]
- Orendorff CJ, Murphy CJ. Quantitation of metal content in the silverassisted growth of gold nanorods. J Phys Chem B 2006;110:3990-4.[LinkOut]
- Jang B, Kim YS, Choi Y. Effects of gold nanorod concentration on the depth-related temperature increase during hyperthermic ablation. Small 2011;7:265-70.[LinkOut]
- Huang X, Tang S, Mu X, et al. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nanotechnol 2011;6:28-32.[LinkOut]
- von Maltzahn G, Park JH, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res 2009;69:3892-900.[LinkOut]
- Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol 2009;13:256-62.[LinkOut]
- Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338:284-93.[LinkOut]
- Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol 2008;108:619-26.[LinkOut]
- Siegel BA, Dehdashti F, Mutch DG, et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J Nucl Med 2003;44:700-7.[LinkOut]
- Liao H, Hafner JH. Gold nanorod bioconjugates. Chemistry of Materials 2005;17:4636-41.[LinkOut]
- Dhar S, Liu Z, Thomale J, et al. Targeted single-wall carbon nanotubemediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc 2008;130:11467-76.[LinkOut]
- Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989;24:148-54.[LinkOut]
- Jang B, Park JY, Tung CH, et al. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011;5:1086-94.[LinkOut]
- Behr TM, Gotthardt M, Becker W, et al. Radioiodination of monoclonal antibodies, proteins and peptides for diagnosis and therapy. A review of standardized, reliable and safe procedures for clinical grade levels kBq to GBq in the Göttingen/Marburg experience. Nuklearmedizin 2002;41:71-9.[LinkOut]
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31.[LinkOut]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 2010;10:36-46.[LinkOut]
- Nahrendorf M, Sosnovik D, Chen JW, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation 2008;117:1153-60.[LinkOut]
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol 2007;19:289-95.[LinkOut]
- Chen WT, Mahmood U, Weissleder R, et al. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther 2005;7:R310-7.[LinkOut]
- Shao X, Zhang H, Rajian JR, et al. 125I-labeled gold nanorods for targeted imaging of inflammation. ACS Nano 2011;5:8967-73.[LinkOut]
Supplementary material
Synthesis of amine-functionalized folate, FA-miniPEG-NH2
For the synthesis of H2N(CH2)3O(CH2)2O(CH2)2O(CH2)3NHBoc, 4,7,10-trioxa-1,13-tridecanediamine (7.5 g, 34.1 mmol) was dissolved in 1,4-dioxane (80 mL), deoxygenated by Ar (g) purging. Then, a solution of Boc2O (3.7 g, 16.95 mmol) in 1,4-dioxane (20 mL) was added slowly. The reaction mixture was stirred at room temperature for 12 h. The solvent was removed, and the resulting yellow oil was purified by silica-gel chromatography (methanol/ dichoromethane/triethylamine = 8/91/1) to produce compound 1 in 30% (3.23g) yield. 1H-NMR (CDCl3, 400 MHz); δ 5.09 (s, 1H), 3.66–3.50 (m, 12H), 3.22 (m, 2H), 2.80 (t, J = 8.0 Hz, 2H), 1.80–1.70 (m, 4H), 1.43 (s, 9H).
For the synthesis of FA-miniPEG-NH2, folate (3.8 g, 8.588 mmol) was dissolved in DMSO (130 mL), deoxygenated by Ar (g) purging. Dicyclohexylcarbodiimide (4.44 g, 21.77 mmol) and pyridine (75 mL) were added and then a solution of H2N(CH2)3O(CH2)2O(CH2)2O(CH2)3NHB OC (3.03 g, 9.456 mmol) in DMSO (20 mL) was added. The reaction mixture was stirred at room temperature for 18 h in the dark. The solid product, dicyclohexylurea, was filtered off, and diethyl ether (2 L) was added slowly. The formed yellow solid was collected and washed with diethyl ether several times.
After evaporation, the resulting compound 2 was dissolved in trifluoroacetic acid (25 mL) and stirred for 2 h. Trifluoroacetic acid was removed under vacuum and diethyl ether was added. A red precipitate formed and was washed with diethyl ether. Finally, compound 3 was isolated in 36% (1.996 g) yield. FAminiPEG- NH2 was identified by HPLC, NMR and ESI-Mass.



