Chemical shift imaging: preliminary experience as an alternative sequence for defining the extent of a bone tumor
Introduction
By providing high contrast resolution, magnetic resonance imaging (MRI) plays a central role in defining the intra-medullary extent of a bone tumor, essential to surgical planning (1). In particular, the signal intensity difference between normal bone marrow containing fatty marrow and a marrow-replacing intra-medullary tumor is best on a non-contrast T1-weighted spin echo (T1SE) sequence. As such, T1SE imaging is indispensable for identifying the intra-medullary extent of a bone tumor (2,3). Other sequences, part of a comprehensive tumor protocol, generally include fluid-sensitive sequences with T2 weighting or short tau inversion recovery (STIR) and contrast-enhanced T1 weighted imaging. Fluid-sensitive sequences have the potential to overestimate tumor extent due to the presence of peri-lesional bone marrow edema which may be similar in signal intensity to a tumor. With gadolinium administration, a tumor typically enhances on a T1 weighted sequence, although areas of non-tumoral tissue, such as peri-lesional inflammation, may also enhance (4), creating the potential for overestimating tumor extent on contrast-enhanced T1 weighted imaging.
An additional T1-weighted option for evaluating the bone marrow has been introduced with chemical shift imaging [CSI, in phase (IP) and opposed-phase (OP) gradient echo sequences]. CSI is a means of differentiating a marrow replacing tumor from abnormalities in the marrow that do not replace marrow fat (5-9). The latter include entities such as red marrow or bone marrow edema, commonly found around bone tumors. CSI is based on the principle that protons attached to water and fat precess with slightly different frequencies; when a voxel contains fat and water, there is an additive effect on the signal in the IP image with at least a 20% loss of signal on the OP image. If the voxel contains a tumor replacing normal fatty marrow, there is only water within the voxel and there is no significant drop in signal (less than 20%) on the OP image compared with the IP image (10).
Hence, we hypothesized that CSI is as accurate as T1SE imaging for determining the extent of a tumor, by providing good contrast between a marrow-replacing tumor and surrounding peri-lesional edema. However, in patients with abundant hematopoietic marrow, such as pediatric patients, or patients with a history of anemia, smoking or obesity (11,12), we hypothesized that contrast between a tumor and normal red marrow may potentially be insufficient using a T1SE sequence, rendering the identification of tumor borders potentially difficult. The purpose of this study was to investigate whether CSI is a useful addition to a tumor protocol for determining the intra-medullary extent of a skeletal tumor.
Methods and materials
Overview
This retrospective study was HIPAA compliant and approved by the institutional review board (IRB) of our institution. Informed consent was waived. The imaging of subjects with histologically-proven tumors was reviewed, for intramedullary tumor extent. Measurements of tumor extent were recorded for each tumor on multiple sequences and compared. The amount of red marrow was also evaluated and its impact on tumor measurements was sought.
Study population
Fifty-one consecutive subjects who had undergone an MRI for evaluation of an untreated bone tumor between April 2008 and November 2011 were retrieved from the picture archiving and communications system (PACS) at our institution. Inclusion criteria were patients with biopsy-proven histologies, a bone lesion measuring at least 2 cm in size in at least one plane on MRI and an MRI protocol which included T1SE sequences, IP and OP sequences, fluid-sensitive sequences and contrast-enhanced T1-weighted imaging. For malignant lesions, histologic proof of free margins was necessary for proving tumor extent by MRI. For benign lesions, histologic proof was given by percutaneous biopsy or excision. Exclusion criteria were patients with incomplete imaging, lack of histologic proof, lesions less than 2 cm in at least one plane on MRI, and lesions with a predominantly extra-skeletal soft tissue mass. Following exclusion, 23 subjects were identified (17 patients with tumors of the appendicular skeleton and 6 patients with tumors of the axial skeleton). Of the 23 subjects, 21 were malignant tumors [11 osteosarcoma, 1 lymphoma, 2 Ewing’s sarcoma, 1 Giant Cell Tumor, 6 metastasis (2 breast cancer, 1 renal cell carcinoma, 1 follicular thyroid carcinoma, 1 infantile fibrosarcoma, 1 pleiomorphic myxofibrosarcoma)] and 2 were benign tumors (1 aneurysmal bone cyst and 1 Langerhans Histiocytosis).
Observer procedures
One radiologist with 10 years of experience in musculoskeletal imaging after training reviewed the 23 MRI exams on the PACS system (UV; Emageon, Birmingham, AL, USA). Images were reviewed in the following order: The IP and OP sequences were first reviewed, followed by the non-contrast T1SE sequence, the contrast-enhanced T1 weighted sequence and then the fluid sensitive sequences. Regions of intra-medullary tumor were defined as follows: any region within the bone marrow with a drop in signal less than 20% on OP compared with IP gradient echo images, any region of low signal intensity less than adjacent skeletal muscle on T1 SE imaging, any region with signal intensity greater than normal fat-suppressed fatty marrow on fluid-sensitive imaging, and any region showing contrast enhancement on the post-contrast T1 weighted images. For the chemical shift images, the signal on the IP and OP images was measured using a region of interest (ROI) localized to an area in the bone marrow showing low signal intensity on the IP image; a similar ROI was placed at the same location on the OP image. The ROI was drawn to encompass as much of the signal abnormality as possible, with exclusion of adjacent bone cortex and soft tissues. The intra-medullary extent of each tumor was measured in millimeters, disregarding the soft tissue components, in the same plane on each sequence. In the extremities, the observer recorded the maximal cranio-caudal extent of the tumor on each sequence. In the axial skeleton, the observer recorded the antero-posterior and the medio-lateral extent of the tumor for each bone that was involved and for every slice (for example, if the tumor involved the iliac bone and the sacrum, each bone was recorded separately on every imaging slice that it was identified).
Moreover, because one of the goals of the study was to investigate whether abundant hematopoietic marrow has an impact on measuring the extent of a tumor, the observer graded the amount of red marrow in the bone containing tumor using a ROI with a 4 point grading scale: 0 (no red marrow seen), 1 (less than 50% red marrow in the bone), 2 (between 50% and 75% red marrow in the bone) and 3 (between 75% and 100% red marrow in the bone). The degree of red marrow presence was recorded using the T1SE sequence and identified as an ill-defined region of intermediate signal intensity greater than skeletal muscle within the bone marrow (13).
MRI protocol
MRI was performed on 1.5 Tesla (8 patients/118 measurements) or 3 Tesla (15 patients/35 measurements) systems and included the following protocol: non-contrast T1SE imaging (spin echo, typical TR/TE 400-500/10-20 for 1.5T, typical TR/TE 800/20 for 3T), fat-suppressed T2-weighted imaging (spin echo, TR/TE 4,000-5,000/60-80 for 1.5T, TR/TE 6,000/60 for 3T), CSI (gradient echo, IP TR/TE 114-220/4.7 msec and OP TR/TE 114-220/2.3 msec for 1.5T, IP TR/TE 130-217/2.3 or 4.5 ms and OP TR/TE 217-270/1.1 or 3.6 for 3T) and pre- and post-contrast fat-suppressed T1-weighted imaging (gradient echo, TR/TE 5.9/1.9 for 1.5T and 6.3/1.5 for 3T).
Statistical analysis
All data were stored on a spreadsheet (Excel 2010, Microsoft, Seattle, WA, USA) and analysis was performed using SPSS software package (version 18.0, SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to evaluate the normality of distribution of continuous variables. Normally distributed variables were compared between groups using a paired Student’s t-test and the results were expressed as mean ± standard deviation (SD). The Wilcoxon signed-rank test was used for comparing nonparametric variables. The Spearman’s rank correlation coefficient was used to evaluate the correlation between the percentage of bone marrow involvement and the difference in tumor size measurements by different sequences. A probability level of 0.05 was accepted as statistically significant.
Results
For the 17 tumors in the appendicular skeleton (extremities), the total number of measurements performed was 17 for every sequence (in the craniocaudal dimension). The mean age was 31.12 (range, 7-79) years old. Average lesion size was 104.22 mm (range, 35.44 -332.39 mm) and the tumors involved 7 femurs, 4 tibias, 2 fibulas, and 4 humeri.
For the 6 axial tumors, the total number of measurements performed was 136 for every sequence. There were 1 benign (Langerhans Histiocytosis) for a total of 8 measurements per sequence and 5 malignant lesions (3 Osteosarcomas, 1 metastatic pleiomorphic myxofibrosarcoma, 1 Ewings sarcoma) for a total of 128 measurements per sequence. The mean age was 23.83 (range, 9-73) years old. Average lesion size was 49.4 mm (range, 0-150.7 mm). The tumors involved 5 iliac bones, 3 ischial bones, 2 pubic bones, one sacral bone, one scapula, and one rib.
Measurement differences for the intra-medullary tumor extent are shown in Tables 1 and 2, for appendicular and axial tumors, respectively. For subjects with tumors in the appendicular skeleton, significant measurement differences were found between all sequences, except between T1SE and CSI (P>0.05, mean difference of 1.7 mm between sequences). For subjects with axial tumors, measurements, on average, were again not significantly different between T1SE and CSI sequences (P>0.05, mean difference 4.2 mm). Comparison between measurements obtained on T1SE and other sequences in the axial skeleton yielded significant differences.

Full table

Full table
The abundance of red marrow in each bone was measured on the bone where tumor was identified; as expected, red marrow was more abundant in the axial skeleton than in the extremities. In the extremity bones, 8 locations had red marrow grade 0, 8 locations had grade 1 and 1 location had grade 2. In the axial bones, 10 locations had red marrow grade 0, 44 locations had grade 1, 0 locations had grade 2, and 82 locations had grade 3.
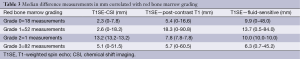
An examination of the average mean difference of the measurements between T1SE and other sequences (CSI, contrast-enhanced T1 and fluid-sensitive) was made according to the abundance of red marrow. Table 3 shows the mean difference in measurements correlated to the bone marrow grading. As red marrow grade in the bone increased, there was an increased measurement difference observed between T1SE and CSI sequences; differences between T1SE and contrast-enhanced T1SE as well as T1SE and fluid-sensitive sequences were variable with grade. Figure 1A-C highlights the differences in tumor measurements in the skeleton by different sequences compared to red marrow grade.
Full table
Discussion
A chemical shift sequence with in-phase and OP gradient echo imaging has emerged as an important technique for differentiating a marrow-replacing tumor from non-neoplastic marrow abnormalities (5-9), such as bone marrow edema or red marrow. As such, high contrast resolution is expected for identifying the extent of a tumor on CSI sequences, by distinguishing areas of tumor from surrounding peri-lesional edema and red marrow. To the best our knowledge, this the first study identifying CSI as an alternative technique for determining the intra-medullary extent of a skeletal tumor.
A non-contrast T1SE sequence has been shown to have excellent sensitivity for detecting the borders of a bone tumor. With T1SE, bone tumors have significantly different signal characteristics compared with native fatty marrow and classically exhibit well-defined morphology against the surrounding normal marrow (1-3). Fluid sensitive sequences tend to overestimate the tumor extent, mainly due to peri-lesional edema that can sometimes be challenging to distinguish from the tumor (1). Often, as part of a routine tumor protocol, contrast-enhanced T1 weighted imaging is performed and can be used for defining tumor extent (14), but contrast enhancement is known to occur in non-neoplastic regions as well as neoplastic regions, such as areas of peri-lesional inflammation (4). In fact, Seeger et al. concluded that Gadolinium does not help to define the intra-medullary extent of osteosarcomas of the extremities. Several articles have instead established T1SE sequences as sufficient for defining the intra-medullary extent of bone tumors (1-3). In this study, we show that CSI with IP and OP sequences is an alternate technique for identifying tumor extent. CSI has been shown to differentiate a fracture with surrounding bone marrow edema from a pathologic fracture with surrounding tumor in vertebral fractures (6-8). Similarly, Disler et al. showed that IP and OP sequences are helpful in distinguishing a bone marrow replacing lesion from red marrow and bone marrow edema in other areas of the body (5).
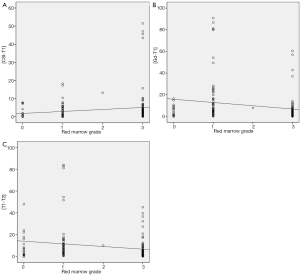
In extremity tumors, CSI measurements were nearly identical with measurements made on the T1 SE images, suggesting little added value for the CSI sequence. As expected commonly in the extremities of adults, red marrow was scarce in the study population. However, in the axial skeleton, where red marrow was more abundant, measurements on CSI and T1SE sequences differed increasingly as red marrow grade increased; for example, the maximal difference in measurements obtained on these two sequences was as high as 5.1 cm when red marrow was highly abundant. The latter finding suggests the possibility that the reader had difficulty in accurately measuring the borders of the tumor when dense red marrow was present in the surrounding bone (Figure 2). Figure 3 highlights this potential challenge with an example of histologically-proven extension into the sacrum, clearly identified by CSI, but meeting equivocal criteria for tumor extension by T1SE imaging. Furthermore, as red marrow abundance increased in the bone, measurements on T1SE and contrast-enhanced T1 imaging became more similar, again potentially suggesting the possibility that T1SE overestimates the tumor extent by including surrounding red marrow that enhances on post-contrast sequences (15).
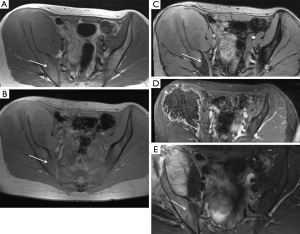
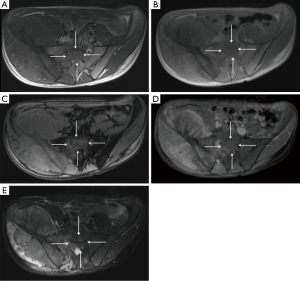
Our study has some limitations. First, the study design would be improved by a prospective section by section comparative analysis between the resected specimen and MRI sections; the latter may potentially show that CSI measurements are superior to T1SE imaging in patients with abundant red marrow surrounding a bone tumor. Conversely, although no direct comparison to histologic sections was made in this study, all prospective MRI readings used available MRI sequences (including CSI) to determine tumor extent and in all cases, the surgical margins were clear, indicating that prospective MRI measurements with CSI in the protocol accurately defined the tumor measurements. Other limitations of this study include a relative small number of patients, but given the size of the tumors and number of bones involved, there were over 150 measurements in the study sample. Finally, a heterogeneous group of histologies were included in this study, because the primary purpose was to determine tumor extent, rather than character of the tumor. It is possible that some histologies may show variable signal on MRI sequences that creates an inconsistent challenge for defining the intra-medullary tumor compared with surrounding edema or red marrow.
In conclusion, CSI is an alternative technique to T1SE imaging for defining the intra-medullary extent of a bone tumor. Our results introduce doubt regarding the T1SE measurements of intra-medullary tumor extent when there is abundant red marrow in the surrounding bone. In such areas as the pelvis where there is inherently increased red marrow compared with the extremities, in pediatric patients whose bone marrow has not yet converted to fatty marrow, and in patients with histories of anemia, obesity or smoking, the T1SE sequence may be lacking. In the future, CSI measurements must be correlated with histologic sections to unequivocally confirm any potential benefit of this sequence for determining intra-medullary tumor extent.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Onikul E, Fletcher BD, Parham DM, et al. Accuracy of MR imaging for estimating intraosseous extent of osteosarcoma. AJR Am J Roentgenol 1996;167:1211-5. [PubMed]
- Gillespy T 3rd, Manfrini M, Ruggieri P, et al. Staging of intraosseous extent of osteosarcoma: correlation of preoperative CT and MR imaging with pathologic macroslides. Radiology 1988;167:765-7. [PubMed]
- Seeger LL, Widoff BE, Bassett LW, et al. Preoperative evaluation of osteosarcoma: value of gadopentetate dimeglumine-enhanced MR imaging. AJR Am J Roentgenol 1991;157:347-51. [PubMed]
- Erlemann R, Reiser MF, Peters PE, et al. Musculoskeletal neoplasms: static and dynamic Gd-DTPA--enhanced MR imaging. Radiology 1989;171:767-73. [PubMed]
- Disler DG, McCauley TR, Ratner LM, et al. In-phase and out-of-phase MR imaging of bone marrow: prediction of neoplasia based on the detection of coexistent fat and water. AJR Am J Roentgenol 1997;169:1439-47. [PubMed]
- Zampa V, Cosottini M, Michelassi C, et al. Value of opposed-phase gradient-echo technique in distinguishing between benign and malignant vertebral lesions. Eur Radiol 2002;12:1811-8. [PubMed]
- Eito K, Waka S, Naoko N, et al. Vertebral neoplastic compression fractures: assessment by dual-phase chemical shift imaging. J Magn Reson Imaging 2004;20:1020-4. [PubMed]
- Erly WK, Oh ES, Outwater EK. The utility of in-phase/opposed-phase imaging in differentiating malignancy from acute benign compression fractures of the spine. AJNR Am J Neuroradiol 2006;27:1183-8. [PubMed]
- Swartz PG, Roberts CC. Radiological reasoning: bone marrow changes on MRI. AJR Am J Roentgenol 2009;193:S1-4, Quiz S5-9.
- Zajick DC Jr, Morrison WB, Schweitzer ME, et al. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology 2005;237:590-6. [PubMed]
- Shellock FG, Morris E, Deutsch AL, et al. Hematopoietic bone marrow hyperplasia: high prevalence on MR images of the knee in asymptomatic marathon runners. AJR Am J Roentgenol 1992;158:335-8. [PubMed]
- Poulton TB, Murphy WD, Duerk JL, et al. Bone marrow reconversion in adults who are smokers: MR Imaging findings. AJR Am J Roentgenol 1993;161:1217-21. [PubMed]
- Vande Berg BC, Malghem J, Lecouvet FE, et al. Magnetic resonance imaging of normal bone marrow. Eur Radiol 1998;8:1327-34. [PubMed]
- Verstraete KL, Lang P. Bone and soft tissue tumors: the role of contrast agents for MR imaging. Eur J Radiol 2000;34:229-46. [PubMed]
- Dwek JR, Shapiro F, Laor T, et al. Normal gadolinium-enhanced MR images of the developing appendicular skeleton: Part 2. Epiphyseal and metaphyseal marrow. AJR Am J Roentgenol 1997;169:191-6. [PubMed]


