Roles of ultrasound and power Doppler ultrasound for diagnosis of Hashimoto thyroiditis in anti-thyroid marker-positive euthyroid subjects
Introduction
Chronic lymphocytic thyroiditis, or Hashimoto’s thyroiditis (HT), is an autoimmune disease related to and mediated by anti-thyroid autoantibodies (1-4). It is a common disease that may affect up to 2% of all women in the population that commonly occurs between the ages of 30 and 50 years (1). The diagnosis of HT is based on clinical findings and the results of biochemical and serological tests. HT is one of the most common causes of hypothyroidism, which may be subclinical in up to 90% of patients. However, mild thyrotoxicosis symptoms may be observed, especially during the early phase of the disease. Mild hypothyroidism symptoms may be present in 20% of patients when first seen or commonly develop over several years (3). Serological markers are necessary for the diagnosis of HT and for the differential diagnosis of other autoimmune conditions. In addition to the clinical and laboratory findings, ultrasound (US) is a commonly used method for the diagnosis of HT. The sonographic appearance of HT involves a wide range of findings. Commonly, the thyroid gland is often diffusely enlarged, the parenchymal echogenicity is coarsened, more hypoechoic than normal, and often hypervascular on color Doppler sonography (5). A micronodular pattern has been shown to be highly supportive of a diagnosis of HT (6).
The present study was performed to investigate the sonographic findings that may lead to a diagnosis of HT in anti-thyroid marker-positive patients with normal hormone levels (i.e., euthyroid subjects with HT).
Materials and methods
Subjects
After receiving the approval of the local Ethics Committee of our institution and informed consent for the examinations, 40 premenopausal euthyroid patients with a median age of 32 years (range, 20-44 years) with normal levels of free triiodothyronine (FT3) and free thyroxine (FT4) and elevated anti-thyroid peroxidase (TPO) antibodies were enrolled in the study. Exclusion criteria were any chronic disease or acute inflammatory conditions, any medications that may alter thyroid hormones, history of thyroid dysfunction, thyroidectomy, radioiodine therapy, and pregnancy.
A control group of 46 healthy individuals with a median age of 29 years (range, 18-43 years) comprised randomly selected volunteers from among the hospital staff who were unaffected by any disease or acute inflammatory condition at the time of the investigation. None had been prescribed or were using any medications. They were all non-pregnant and were euthyroid in terms of FT3 and FT4 levels with thyroid-stimulating hormone (TSH) levels between 0.5-5 µIU/mL. The same investigation procedures were applied for both the control and study groups.
Sonographic analysis
Thyroid sonographic examinations were performed by the same radiologist with 20 years’ experience in US, who was blinded to the clinical and laboratory status of the subjects during the study. A high-resolution fully equipped US and Doppler US device (Philips HDI 5000; Philips Healthcare, Bothell, WA, USA) and a 7-12 MHz broadband linear array transducer was used for the examinations.
The examinations included basic morphometric, morphological grayscale imaging, as well as power Doppler evaluation of the thyroid gland. Morphometric investigation involved measurement of the thyroid gland dimensions in three axes. The volume of the thyroid lobe was calculated as (anteroposterior × mediolateral × craniocaudal diameters) ×0.479 (7). The total thyroid volume was determined as the sum of the thyroid lobes. Grayscale morphological investigation of the thyroid included evaluation of echogenicity, nodularity, septations, undulation of the margins, and reactive lymph nodes. The vascularity of the gland was investigated by power Doppler imaging.
On grayscale, the presence of nodularity of the thyroid was noted and categorized as macronodular, micronodular, or mixed type. The location of nodularity, if present, was also considered and classified as subcapsular, inner, or both. Undulation of the thyroid gland margins and the presence of septation(s) inside the gland were investigated. We also examined the presence of cervical lymph nodes at infrathyroidal and pretracheal regions.
The vascularity of the thyroid gland was determined by power Doppler imaging using the same standard thyroid setup parameters of the device in each patient. A scale consisting of increased, normal, and decreased vascularity was used for scoring of the gland vascularity on power Doppler imaging.
Statistical analysis
Variable distributions were assessed by the Kolmogorov-Smirnov test. The Mann-Whitney U test was used for comparison of groups. The chi-square test was used for comparison of dichotomous variables. SPSS 15.0 for Windows was used for statistical analyses. Data are expressed as means ± standard error of mean. In all analyses, P<0.05 was taken to indicate statistical significance.
Results
The morphometric parameters of the thyroid gland studied here, including the dimensions and the volume of individual lobes as well as the total volume, were not significantly different between the study and control groups (Table 1).
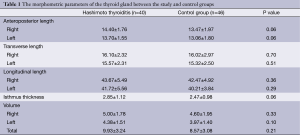
Full table
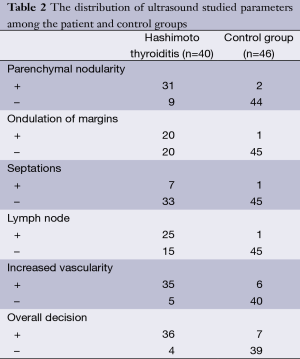
Parenchymal nodularity was detected in 31 of 40 patients (77.5%) and in 4 of 46 (8.6%) subjects in the control group. In the HT group, 14 of the 31 patients with parenchymal nodularity showed micronodularity only, while 17 showed macronodularity or mixed nodularity. Micronodularity and macro- or mixed nodularity were observed in two and two of the four controls with parenchymal nodularity, respectively. The differences between the groups in the presence of nodularity and also the distribution of cases between the subgroups were statistically significant. The undulation of the gland margins was a feature of the disease in half (20 of 40, 50%) of the patients in the HT group. The same finding was detected in one subject in the control group (1 of 46, 2.2%). Septations inside the gland were present in 7 of 40 (17.5%) patients in the HT group and 1 of 46 subjects in the control group (2.2%). Infrathyroidal and/or pretracheal reactive lymph nodes were encountered in 25 of 40 patients (62.5%), compared to only one subject in the control group (2.1%). On power Doppler imaging, increased vascularity was found in 35 of 40 (87.5%) patients and in 6 of 46 (13%) control subjects. The grayscale and power Doppler images of some patients and control subjects, including false positive and false negative examples, are shown in Figures 1-5.
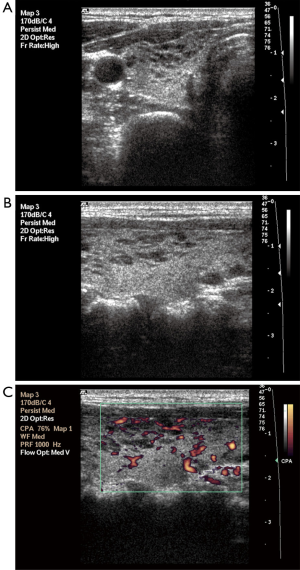
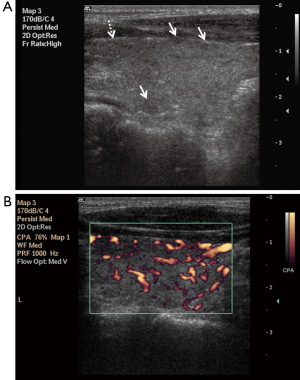

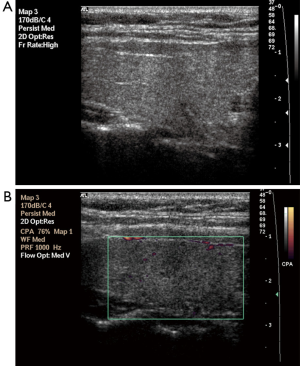
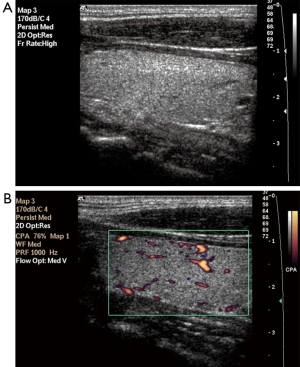
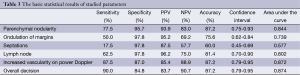
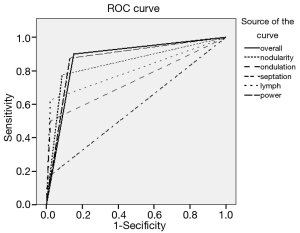
The use of all parameters together yielded a sensitivity of 90%, specificity of 84.8%, positive predictive value (PPV) of 83.7%, negative predictive value (NPV) of 90.7%, and accuracy of 87.2% for diagnosis of HT. The distributions of parameters studied among the patient and control groups are presented in Table 2 and basic statistical results are shown in Table 3. The receiver operating characteristic (ROC) curves of the parameters are shown in Figure 6.
Full table
Full table
Discussion
HT is an autoimmune thyroid disease that is characterized by thyroid gland infiltration by T and B cells. The activated B cells secrete anti-thyroid autoantibodies. The cytotoxic T cells are largely responsible for destruction of the thyroid parenchyma, leading to initial thyrotoxicosis followed by hypothyroidism. HT may clinically present as painless diffuse goiter often accompanied by hypothyroidism and autoantibodies (4). However, the disease is not invariably goitrogenic in all patients and may affect the thyroid gland without changing the morphometric parameters, such as the dimensions or volume, as observed in the present study.
Previous studies have indicated that autoantibodies may remain absent in 13% or may be low in 17% of patients with HT, and may also be present in 2-20% of the normal population without thyroid disease (8). In this gray zone of disease and normality, US and Doppler US may be used as additional tools for the diagnosis of HT. Acar et al. (2) reported that an antibody-positive healthy subpopulation without any symptoms or laboratory disorders demonstrated grayscale and hemodynamic US features similar to those of HT patients with hypothyroid status.
Anderson et al. (8) reported that the sensitivity and specificity are improved by combining US with clinical and serological parameters. Previous studies demonstrated that in HT, the thyroid is hypoechoic with a heterogeneous echotexture, echogenic septations, and micronodulation on sonography. Hypoechoic areas result from focal lymphocytic infiltration and the echogenic septations occur secondary to fibrosis inside the parenchyma (5,6). Nodule development inside the thyroid gland is the most commonly reported feature of HT (3). Some investigations have described the sonographic appearance of focal nodules that proved to be thyroiditis in patients with known lymphocytic thyroiditis. These have been called pseudotumors, which are detected on US typically with hypoechoic lesions with ill-defined margins (9). Yarman et al. (10) compared the results of US and scintigraphy of patients and reported that the number of nodules detected on scintigraphy was greater than that on US examination. They considered that the difference in nodularity between thyroid scanning (74.9%) and sonography (60.4%) was due to pseudonodularity in HT. On the other hand, Erdogan et al. (3) reported that nodule formation (solitary or multiple) was the most common finding on US in their patients, but physical examinations of these patients revealed much lower rates of thyroid nodules. This discrepancy was probably due to pseudonodule formation in HT. Generally, these nodules range in size from 1 to 7 mm and have been reported as micronodules (6). Yeh et al. (6) and Anderson et al. (8) considered the presence of micronodules as highly diagnostic of HT. The PPV for micronodules in diagnosis of HT has been reported to be up to 94.7%. In the present study, nodularity was also a prominent feature among the grayscale findings with 95.7% specificity, 93.9% PPV, and 87.2% accuracy.
At the initial phase of this disease, US shows hypoechogenic areas with irregular poorly defined margins, especially in the subcapsular zones, followed by pseudonodule formation in the central region of the thyroid gland (11). Subcapsular nodules contribute to undulation of the thyroid gland margins, which was observed in half of the patients but in only one subject in the control group. Hence, the specificity and PPV of margin undulation were 97.8% and 95.2%, respectively. Septum formation was not frequently encountered in our study group. Sometimes, the echotexture changes in HT may be subtle or may be difficult to assess that may mislead the observer to the decision of normal echogenicity. Image texture analysis of the thyroid has been proposed to be helpful for discriminating the normal and the inflamed parenchyma (12-14).
Lymph node involvement at infrathyroidal and pretracheal areas has been associated with pathological conditions of the thyroid, as these areas are the preferential sites of thyroid lymphatic drainage (11). Yamashiro et al. (11) reported cervical chain lymph nodes at level VI in 28 of 38 patients (73.6%), all of whom presented reactive features (ovoid, with hyperechogenic central hilum). Brancato et al. (15) suggested that an increased number of benign hyperplastic neck nodes, especially at levels II (upper internal jugular nodes), III (middle jugular nodes), and IV (paratracheal), is a characteristic sonographic finding associated with chronic autoimmune thyroiditis. In the present study, we detected cervical lymph nodes on neck US examination in 62.5% (n=25) of HT patients. However, the presence of lymph nodes in infrathyroidal and pretracheal locations indicated HT with specificity of 97.8% and PPV of 96.2%.
On Doppler US, the thyroid parenchyma may vary from slightly to markedly hypervascular in HT. This increased vascularity seems to be associated with the development of hypothyroidism, which may be due to the trophic stimulation of TSH (8,16,17). Doppler examination in the early phases may show a pattern of diffuse hypervascularization similar to Graves’ disease. In the later stages of the disease, Doppler examination shows a reduction in size of the thyroid gland with diffuse heterogeneity due the intense fibrosis and avascularity (11). We observed increased thyroid gland vascularity in 85% (n=34) of our patients. Despite the typical finding of hypervascularity with diffuse HT, in many studies the vascularity of nodular HT was variable (2). This condition may be associated with different stages of thyroiditis.
Kim et al. (18) suggested that for identification of diffuse thyroid disease, a combination of three sonographic features (abnormal sonographic features include echogenicity, echotexture, vascularity, AP diameter, and glandular margin on real-time sonography) has high sensitivity and specificity compared with the use of only two sonographic features of diffuse thyroid disease. According to their study, the present sonographic classification system showed high efficacy and accuracy for the identification of asymptomatic diffuse thyroid disease. In the present study, use of all of the parameters together yielded high sensitivity and high accuracy for the diagnosis of HT.
Conclusions
US and power Doppler US are helpful diagnostic tools in the diagnosis of HT in anti-thyroid marker-positive patients with normal thyroid hormone levels. In addition to grayscale parameters, the use of power Doppler US and investigation of the presence of paratracheal lymph nodes increased the sensitivity and accuracy of US.
Disclosure: The authors declare no conflicts of interest.
References
- Yildirim D, Gurses B, Gurpinar B, Ekci B, Colakoglu B, Kaur A. Nodule or pseudonodule? Differentiation in Hashimoto’s thyroiditis with sonoelastography. J Int Med Res 2011;39:2360-9. [PubMed]
- Acar T, Ozbek SS, Erdogan M, Ozgen AG, Demirel SO. US findings in euthyroid patients with positive antithyroid autoantibody tests compared to normal and hypothyroid cases. Diagn Interv Radiol 2013;19:265-70. [PubMed]
- Erdogan M, Erdem N, Cetinkalp S, Ozgen AG, Saygılı F, Yilmaz C, Tuzun M, Kabalak T. Demographic, clinical, laboratory, ultrasonographic, and cytological features of patients with Hashimoto’s thyroiditis: results of a university hospital of 769 patients in Turkey. Endocrine 2009;36:486-90. [PubMed]
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55. [PubMed]
- Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 2000;10:251-9. [PubMed]
- Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med 1996;15:813-9. [PubMed]
- Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jørgensen T. The association between hypoechogenicity or irregular echo pattern at thyroid ultrasonography and thyroid function in the general population. Eur J Endocrinol 2006;155:547-52. [PubMed]
- Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, Szabunio MM, Hildebolt CF, Mandel SJ, Cronan JJ. Hashimoto thyroiditis: Part 1, sonographic analysis of the nodular form of Hashimoto thyroiditis. AJR Am J Roentgenol 2010;195:208-15. [PubMed]
- Langer JE, Khan A, Nisenbaum HL, Baloch ZW, Horii SC, Coleman BG, Mandel SJ. Sonographic appearance of focal thyroiditis. AJR Am J Roentgenol 2001;176:751-4. [PubMed]
- Yarman S, Mudun A, Alagol F, Tanakol R, Azizlerli H, Oguz H, Cantez S. Scintigraphic varieties in Hashimoto’s thyroiditis and comparison with ultrasonography. Nucl Med Commun 1997;18:951-6. [PubMed]
- Yamashiro I, Saito OC, Chammas MC, Cerri GG. Ultrasound findings in thyroiditis. Radiol Bras 2007;40:75-9.
- Smutek D, Sára R, Sucharda P, Tjahjadi T, Svec M. Image texture analysis of sonograms in chronic inflammations of thyroid gland. Ultrasound Med Biol 2003;29:1531-43. [PubMed]
- Mailloux GE, Bertrand M, Stampfler R, Ethier S. Local histogram information content of ultrasound B-mode echographic texture. Ultrasound Med Biol 1985;11:743-50. [PubMed]
- Koprowski R, Zieleźnik W, Wróbel Z, Małyszek J, Stępień B, Wójcik W. Assessment of significance of features acquired from thyroid ultrasonograms in Hashimoto’s disease. Biomed Eng Online 2012;11:48. [PubMed]
- Brancato D, Citarrella R, Richiusa P, Amato MC, Vetro C, Galluzzo CG. Neck lymph nodes in chronic autoimmune thyroiditis: the sonographic pattern. Thyroid 2013;23:173-7. [PubMed]
- Sarikaya B, Demirbilek H, Akata D, Kandemir N. The role of the resistive index in Hashimoto’s thyroiditis: a sonographic pilot study in children. Clinics (Sao Paulo) 2012;67:1253-7. [PubMed]
- Onoda N, Kato Y, Seki T, Kurimoto M, Takano K, Ito Y, Sato K. Increased thyroid blood flow in the hypoechoic lesions in patients with recurrent, painful Hashimoto’s thyroiditis at the time of acute exacerbation. Endocr J 2009;56:65-72. [PubMed]
- Kim DW, Eun CK, In HS, Kim MH, Jung SJ, Bae SK. Sonographic differentiation of asymptomatic diffuse thyroid disease from normal thyroid: a prospective study. AJNR Am J Neuroradiol 2010;31:1956-60. [PubMed]


