Established and emerging cardiovascular magnetic resonance techniques for the assessment of stable coronary heart disease and acute coronary syndromes
Introduction
Coronary heart disease (CHD) is a leading cause of death and disability worldwide. In the United States (US) 15.4 million people have CHD costing the US economy $108.9 billion/yr (1) and each year 715,000 have a myocardial infarction (MI) (2); whilst in the United Kingdom (UK) there are an estimated 2 million people with angina costing £9.0 billion/yr (3). In a typical hospital setting a variety of investigations may be used to diagnose CHD, as well as risk stratify the individual and determine the need for coronary revascularization. These may involve anatomical imaging of the coronary arterial tree with computed tomography coronary angiography (CTCA) or invasive X-ray coronary angiography; or assessment for functionally significant coronary artery stenosis with single-photon emission computed tomography (SPECT), stress echocardiography, cardiovascular magnetic resonance (CMR) or positron emission tomography (PET).
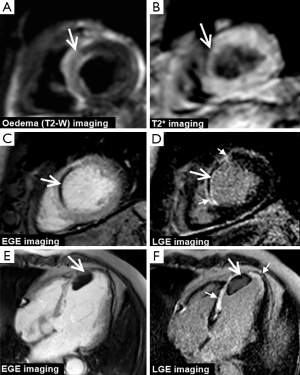
CMR produces high resolution images which can be acquired in any plane and allows the assessment of global and regional cardiac function, myocardial perfusion, myocardial viability, tissue characterisation and proximal coronary anatomy—all within a single study and without the use of ionising radiation. This unique multi-parametric approach leads to a high diagnostic accuracy for the detection of CHD and an important role in the management of both the stable and acute patient. In patients with stable CHD, CMR can detect and localise ischemia, quantify ischemic burden and determine myocardial viability, all of which can be used to risk-stratify patients and guide revascularization (Figure 1). In patients presenting with acute coronary syndromes (ACS), CMR can accurately determine ischemia and infarction and provide prognostic information such as the size and location of MI, the area at risk (myocardial edema) and the presence or absence of microvascular obstruction (MO), intramyocardial hemorrhage (IMH) or sequelae such as left ventricular (LV) thrombus (Figure 2).
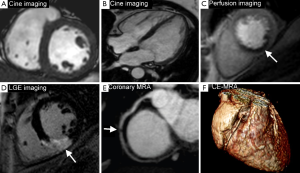
CMR is therefore firmly established in both national and international guidelines, which recommend a variety of investigative strategies for the diagnosis of CHD (4-6). The 2013 European Society of Cardiology (ESC) guidelines on the management of stable CHD (5) give a class I recommendation for non-invasive stress testing and recommend CMR as an imaging option for the initial diagnostic assessment of angina. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines give CMR a class IIa recommendation for the investigation of those with intermediate to high pre-test probability of obstructive CHD in those physically able to exercise but with an ECG which would be un-interpretable during an exercise test; and class IIb in those intermediate to high risk unable to exercise (4). There is also a role for ischemia and viability testing with CMR in those with known CHD and after MI, particularly in those with multi-vessel disease (7). The ACCF/AHA gives CMR a class I recommendation in those with known CHD of unclear physiological significance considered for revascularization and the ESC guidelines give non-invasive stress imaging IIa classification for this indication (5).
This article will focus on the rapidly evolving role of the multi-parametric CMR examination in the assessment of patients with stable and unstable CHD.
CMR for the investigation of stable CHD
CMR is an established method for demonstrating myocardial ischemia and in some UK and European centres has become the preferred investigation for patients with suspected stable angina. A CMR study for this purpose takes between 30 and 60 minutes and typically includes cine images in multiple planes for assessment of LV volumes and function, stress and rest perfusion for myocardial ischemia and late gadolinium enhancement (LGE) for delineation of scar and assessment of viability.
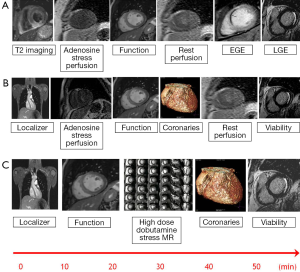
Stress assessment with CMR for myocardial ischemia can be performed with vasodilatory or inotropic stress agents. Vasodilatory stress with adenosine, regadenoson (and less commonly dipyridamole or nicorandil) uses gadolinium based contrast agents to demonstrate myocardial hypoperfusion. Dobutamine stress CMR, like stress echocardiography, induces wall motion abnormalities in the presence of functionally significant coronary stenoses without the need for a gadolinium based contrast agent (although first pass perfusion can be performed at peak stress for additional value). Typical multi-parametric CMR protocols can be seen in Figure 3.
Vasodilatory stress CMR has high diagnostic accuracy for the detection of CHD and a recent meta-analysis of 37 studies demonstrated a combined sensitivity of 89% (95% CI: 88-91%) and specificity of 76% (95% CI: 73-78%) (8). The largest prospective randomized controlled trial, the CE-MARC study, which was not included in the meta-analysis, demonstrated similar results and comprehensively established superiority over SPECT with a higher sensitivity (87% vs. 67%, P<0.0001) and negative predictive value (91% vs. 79%, P<0.0001) but similar specificity (83% vs. 83%, P=0.916) and positive predictive values (77% vs. 71%, P=0.061) (9,10). A recent pre-specified CE-MARC gender sub-analysis has shown that in terms of sensitivity, CMR outperformed SPECT in both males and females, whereas the sensitivity of SPECT in females was significantly worse than in males (11).
Like CE-MARC, the subsequently published multi-centre MR-IMPACT II trial also showed a greater sensitivity of CMR compared to SPECT (67% vs. 59%, P=0.024) but a lower specificity (61% vs. 72%, P=0.038) (12). However in this trial only the perfusion components of the CMR examination were analysed and as a result, diagnostic accuracy was comparatively lower. This may also be explained by the multicenter, multivendor, non-standardized pulse sequence trial design of MR-IMPACT II with reporting performed by an independent core laboratory without clinical details, and emphasizes the incremental value of reporting imaging studies in their clinical context and with experience and knowledge of the techniques used.
Whilst both CE-MARC and MR-IMPACT II assessed the ability for CMR and SPECT to detect inducible myocardial perfusion deficits with adenosine stress, CE-MARC also evaluated the incremental value of the addition of infarction detection with LGE, cine imaging for regional ventricular function and magnetic resonance angiography (MRA) for coronary artery anatomy. The value of combining such components in one single multi-parametric CMR examination added to the increased specificity in the CE-MARC trial. Indeed this issue has been examined in small scale studies with ventricular function and LGE improving the specificity and diagnostic accuracy above the stress perfusion examination alone. The clinical utility of imaging coronary artery anatomy by MRA within already lengthy protocols however still remains to be established (13). In CE-MARC the overall diagnostic accuracy did not alter whether or not the results of the MRA were included in the analysis. Other investigators have evaluated the effect of adding coronary MRA to stress perfusion CMR on diagnostic performance; when compared to invasive pressure-wire derived fractional flow reserve (FFR) at 1.5 T there was no significant improvement in diagnostic accuracy (14).
Whilst the CE-MARC study proved the superiority of CMR over SPECT in terms of diagnostic accuracy of CHD detection, questions were raised over the availability and cost benefit of the technology (15). Subsequent health economic analysis demonstrated that a diagnostic strategy which includes CMR is cost effective falling between the lower and upper limits thresholds (£20-30,000) per quality adjusted life year (QALY) as defined by National Institute for Health and Care Excellence (NICE) (16). Furthermore the cost effectiveness of CMR has been corroborated in other international models against both direct to invasive coronary angiography and SPECT, although direct referral to invasive coronary angiography may be more cost effective in those with a high pre-test probability of having underlying CHD (17,18).
Inotropic stress CMR with dobutamine for the detection of significant CHD relies on the induction of wall motion abnormalities and therefore evaluating a later stage of the ischemic cascade than perfusion imaging. Nevertheless dobutamine stress CMR also has a high diagnostic accuracy for the detection of CHD with one meta-analysis of 14 studies showing a pooled sensitivity of 0.83 (95% CI: 0.79-0.88) and specificity of 0.86 (95% CI: 0.81-0.91) (19). One single centre study demonstrated dobutamine stress CMR to be superior to dobutamine stress echocardiography (DSE) with sensitivity of 86% vs. 74%, P<0.05 and specificity 86% vs. 70%, P<0.05, although this benefit of dobutamine stress CMR above DSE was limited to those with suboptimal echocardiographic images (20). In terms of prognostic value, those with a negative DSCMR have an excellent prognosis with an event rate of only 1.2% in the first year after the test (21-23), which is similar to that published annual event rate of 1.3% of a negative DSE (24). Dobutamine stress CMR has been demonstrated to be extremely safe with a comparable safety profile to DSE (25,26).
Pushing the boundaries: improving technology
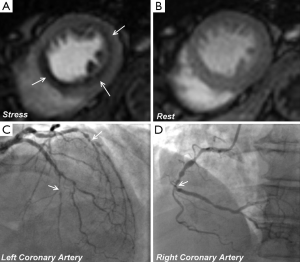
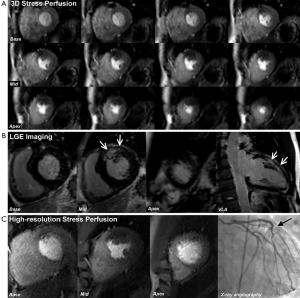
Since the inception of the CE-MARC and MR-IMPACT II studies, which used perfusion sequences with an in-plane spatial resolution of 2-3 mm, there have been major advances in CMR technology. Notably, there have been improvements in acquisition techniques such as highly accelerated pulse sequences based on spatio-temporal undersampling [for example k-t sensitivity encoding (SENSE) and highly constrained back projection (HYPR)] and improvements in hardware, such as higher field strengths and improved cardiac phase-array coils for higher signal-to-noise (27). Perfusion CMR at 1.5 Tesla (T) using k-t SENSE acceleration to achieve an in-plane spatial resolution of 1.6 mm has been demonstrated to have a greater overall diagnostic accuracy than standard resolution (2.5 mm) for identifying both single (P<0.001) and multi vessel disease (P=0.002), with an area under the curve (AUC) of 0.93 vs. 0.83; P<0.001 (27). Similarly, diagnostic performance at 3.0 T exceeds that at 1.5 T for both single-vessel disease (AUC: 0.89 vs. 0.70; P<0.05) and multivessel disease (AUC: 0.95 vs. 0.82; P<0.05) (28). Using similar high resolution techniques at 3.0 T can regularly achieve an in-plane spatial resolution of <1.5 mm, which is the basis for improved detection of subendocardial ischemia (Figure 4), and this advance is now beginning to make the transition into clinical practice (30).
Conventional stress perfusion CMR images are typically acquired in three short axis slices to assess 16 of the 17 segments in the AHA/ACC model (excluding the apical cap). Faster image acquisition also allows 3-dimensional (3D) whole heart myocardial perfusion imaging with full LV coverage and therefore overcomes assumptions made about the myocardium between slices seen with the conventional approach (Figure 5) (27). An additional advantage of 3D perfusion CMR is that all the data are acquired in one shot and thus in the same cardiac phase. Two recent studies have validated 3D perfusion CMR against FFR and shown high diagnostic accuracy (31,32). Manka et al. demonstrated 3D perfusion CMR at 1.5 T was found to have a sensitivity, specificity and diagnostic accuracy of 90%, 82% and 87% respectively (31). Jogiya et al. found similar figures of 91%, 90% and 91% respectively at 3.0 T (32). Both of these studies also verified the feasibility and reproducibility of myocardial ischemic burden quantification from 3D data using volumetry of myocardial hypo-enhancement expressed as a percentage of total myocardium. 3D myocardial stress perfusion CMR is therefore a highly promising development with high diagnostic accuracy, with a potential additional role in the assessment and follow-up of total myocardial ischemic burden.
Future clinical direction: current multicenter studies
We are now in an era of comparative effectiveness research. Whilst diagnostic accuracy studies have a role, as clinicians we are more interested in studies that change patient management, outcomes or quality of life. There are several currently recruiting large prospective, multicenter, randomized controlled trials, which are likely to shape the future clinical guidelines, diagnostic pathways and utility of CMR in clinical practice.
The MR-INFORM study is a non-inferiority trial designed to compare the role of adenosine stress perfusion CMR versus that of routine coronary angiography with invasive FFR to guide revascularization decisions in patients with stable angina and intermediate to high likelihood of CHD. The primary outcome measure is the occurrence of major adverse cardiovascular events (MACE) at 1 year and it will establish the safety of using stress perfusion CMR as the sole determinant of patient management (33).
CE-MARC-2 is a multicenter, three-arm parallel group, randomized controlled trial designed to compare the management strategy of CMR vs. NICE CG95 guidance (6) vs. AHA/ACC SPECT appropriateness criteria (34), for the investigation of patients with stable chest pain of suspected cardiac origin. Patients with a pre-test likelihood of between 10-90% are randomized to have either multi-parametric CMR, SPECT or follow NICE guidance (patients with low pre-test probability of underlying CHD (10-29%) undergoing CTCA; intermediate (30-60%) non-invasive investigation with SPECT and high pre-test probability (61-90%) direct to coronary angiography). This trial is designed to assess the impact of each of the three strategies in reducing the rates of unnecessary invasive angiography, which is important from a clinical, economic and patient preference perspective. The primary outcome measure is FFR defined unnecessary angiography (FFR >0.8) with the important safety secondary outcome measure of MACE at 1 and 3 years.
The ISCHEMIA trial is a worldwide multicentre randomized controlled trial of >8,000 patients with at least moderate ischemia demonstrated with non-invasive imaging (SPECT, stress echocardiography or CMR). The study is designed to test the hypothesis that in patients with moderate or severe ischemia, a routine invasive strategy with early cardiac catheterisation and revascularization plus optimal medical therapy (OMT) is superior to a conservative management strategy of OMT, with cardiac catheterisation and revascularization reserved for those with an acute ischemic event or refractory anginal symptoms. The primary outcome measure is time to cardiovascular death or non-fatal MI.
Taken together these three studies will influence future clinical guidelines by determining the safety and efficacy of CMR as the primary investigation strategy, potentially reducing rates of unnecessary angiography and establishing whether an invasive strategy (in those with demonstrable significant ischemia) is superior to optimal medical management.
CMR after ACS
The pathophysiology and prognosis of both acute and chronic MI are highly variable. Multi-parametric imaging with CMR has high diagnostic accuracy for the detection of CAD in the assessment of both ST-segment and non-ST-segment elevation ACS (35,36). CMR can uniquely determine the likelihood of functional recovery after revascularization, assess the area of myocardium at risk (and myocardial salvage), differentiate acute from chronic infarction, and demonstrate MO and IMH, as well as being able to detect several sequelae of MI. These individual features may be more powerful surrogate markers of outcome than the traditionally used LV ejection fraction.
Acute MI
After an acute coronary syndrome, LGE imaging confirms the presence of MI, which is seen as hyperenhancement, and can determine its size and location. In acute MI, the distribution volume of extracellular gadolinium-based contrast agents is increased within myocardium due to the destruction of sarcolemmal membranes and abnormal washout kinetics. Similarly, in chronic MI, the presence of replacement fibrotic tissue increases the contrast distribution volume. The resulting differences in contrast distribution between normal and injured myocardium can therefore be used to delineate MI (whether it be acute or chronic) using a T1-sensitive inversion-recovery sequence performed 10-15mins after contrast injection—i.e., LGE imaging (Figures 1,2,5).
Myocardial edema
Following acute MI T2-weighted imaging can be used in ACS to identify myocardial edema (inflammation), which occurs in reversibly ischemic injured myocardium (37). Contrast agents are not required as the myocardial free water content affects paramagnetic properties of the tissue providing intrinsic image contrast, although with relatively low signal-to-noise ratio (SNR) and requires experience to interpret. T2-weighted edema imaging is both sensitive (38) and specific (39) to the timing of an event, thereby differentiating acute from chronic infarction (Figure 2). It therefore also allows delineation of the ‘area-at-risk’ (AAR) in acute infarction and the area of ‘myocardial salvage’ calculated by subtraction of the infarcted area determined by LGE (38). The high signal on edema imaging is persistent for up to 2 weeks after the reversible ischemic insult, the AAR can therefore be measured hours or days after a primary PCI, which makes it an ideal research tool for studies assessing novel antithrombotics and adjuvant techniques for mechanical revascularization.
Microvascular obstruction (MO)
In acute MI, despite successful revascularization therapy, perfusion is not completely restored in up to 30% of patients due to MO. This is seen angiographically as the ‘no-reflow’ phenomenon and is the consequence of capillary necrosis, clogging of small myocardial arterioles with embolic debris, acute inflammation, platelet aggregation and vasospasm.
Contrast enhanced CMR allows accurate depiction of areas of microvascular damage within the core of the infarcted myocardium the extent of which correlates with biochemical markers of infarction (40). In MO gadolinium penetration is impaired and limited to diffusion (41,42) and results in contrast devoid low-signal intensity regions within the high-intensity infarcted areas (Figure 2). This may be imaged with several imaging techniques: first-pass perfusion, early gadolinium enhancement (EGE) imaging at 1 to 2 minutes after contrast injection (Figure 2) and LGE (10-15 mins after injection) (43). Studies have shown that the presence and extent of MO (on EGE or LGE imaging) after acute MI is a strong predictor of adverse ventricular remodelling and clinical outcome, independent of infarct size or LV ejection fraction (LVEF) (44-48). Notably, the presence and extent of MO imaged with LGE imaging (so called ‘persistent’ MO) is the strongest predictor of worse outcomes (49). After acute MI, MO slowly shrinks over the following weeks (rarely persisting beyond 1 month) and is therefore not a feature of chronic infarction.
Intramyocardial haemorrhage (IMH)
Reperfusion of severely ischemic myocardium can lead to IMH within the infarct core caused by extravasation of red blood cells through large gaps in damaged endothelial walls. Deoxyhemoglobin is oxidised to methemoglobin, which causes shortening of the T2 relaxation time due to its paramagnetic properties and magnetic susceptibility effect and therefore, hemorrhage can be detected as areas of dark hypointense signal surrounded by edema (bright signal) on T2-weighted imaging. Several studies have validated the use of T2-weighted CMR imaging to identify IMH in acute MI against histopathological findings (50,51). Furthermore, T2* CMR has also shown potential to detect IMH in the setting of acute MI, with the advantage of better distinction from MO (which is also seen as hypointensity on standard T2-weighted imaging) (52) (Figure 2).
Other sequelae of MI
CMR is superior to echocardiography for the identification of ventricular thrombi, which appear as dark filling defects on EGE or LGE imaging, typically on the endocardial surface of infarcts (53) (Figure 2). CMR is also able to detect other complications of MI including ventricular aneurysm, pseudoaneurysms, ventricular septal perforation and mitral regurgitation. Furthermore the high spatial resolution of CMR allows assessment of right ventricular involvement in acute MI.
Assessment of myocardial viability after myocadial infarction
Ischemic myocardial injury is characterised by the presence of scar in predominantly a subendocardial distribution extending towards the epicardium reflecting the transmural gradient in the vulnerability of the myocardium. The transmural extent of hyperenhancement forms the basis upon which LGE can be used to assess tissue viability. The value of LGE CMR imaging for viability assessment in patients with a chronic CAD or a remote history of MI was established in the landmark study by Kim et al. which demonstrated the relationship between transmural extent of hyper-enhancement and the likelihood of functional recovery after revascularization (54). They established that hyper-enhancement <25% of transmural extent was most likely to confer functional recovery, whilst those segments with hyperenhancement >75% of transmural extent were unlikely to benefit from revascularization-importantly this finding was consistent whether the affected segments were hypokinetic, akinetic or dyskinetic. These findings have subsequently been reproduced and a recent meta-analysis of eleven studies enrolling 331 patients using a 50% transmurality cutoff on LGE reported a sensitivity of 95% (95% CI: 93-97%) and specificity of 51% (40-62%) for predicting functional recovery (55). In the acute phase after MI, interpretation of viability is more difficult as some of the hyperenhancement on LGE imaging may relate to myocardial edema (due to increased extracellular volume) rather than non-viable ‘scar’. Nonetheless, the transmural extent of hyperenhancement on LGE imaging has still been shown to accurately predict contractile recovery after MI and revascularisation even when imaging is performed acutely within the first 7 days (56).
Transmurality of LGE is a stronger predictor of both regional and global functional recovery after revascularization than myocardial wall thickness. Shah et al. studied 201 consecutive patients with wall thinning undergoing revascularization observing increased myocardial wall thickness after revascularization in those segments where the LGE was limited to <25% (4.4 mm increasing to 7.5 mm after revascularization, P<0.001) (57). Furthermore in patients with chronic LV systolic dysfunction due to CHD, the transmural extent of LGE has been shown to be the most sensitive technique for the assessment of viability compared to end diastolic wall thickness and wall thickening during low dose dobutamine stress (58). Nevertheless myocardial viability can be assessed with low dose dobutamine (5-10 mcg/kg/min) with any segment considered viable if there is a 2 mm or more demonstrable increase in systolic wall thickening (59). Inotropic reserve assessed by low dose dobutamine has significantly higher specificity (91%) (55) suggesting a combination of the two techniques might improve diagnostic performance.
Prognostic value of CMR in CHD
Currently SPECT remains the most widely performed non-invasive test for myocardial ischemia internationally and provides a wealth of prognostic information gained in over 30 years of experience with the technology. Emerging evidence suggests CMR will be as good, at prognostication, which is unsurprising since the technology assesses the same stage of the ischemic cascade but with higher spatial resolution allowing detection of more subendocardial ischemia and infarction. One recent large meta-analysis of 19 studies and over 11,000 patients showed a negative CMR was associated with only 0.8% annual event rate at 32 months follow-up (vs. 4.9% event rate in those with a positive test; P<0.0001) (60) which is consistent with the reported annual event rate for a negative SPECT (61). This benefit was observed equally whether undergoing vasodilatory stress or dobutamine stress. More recent data from a large prospective cohort of consecutive patients undergoing adenosine stress perfusion have corroborated this prognostic value at an intermediate term follow-up period (4.2±2.1 years) showing that the presence of a reversible perfusion defects was associated with a threefold increase in cardiac death (P<0.0001) and nonfatal MIs (P=0.001) (62).
The presence of LGE has been demonstrated to be associated with an increased mortality risk in both symptomatic (63) and asymptomatic patients (58) without known previous MI. In patients with chronic ischemic cardiomyopathy, LGE scar size independently predicts both death and sustained ventricular arrhythmia in those with preserved (64) and severely impaired LV function (65,66). One meta-analysis demonstrated the presence of LGE in CHD to be associated with a fourfold increase in the hazard ratio of both mortality and MACE, with each incremental gram of scar associated with a 4% increase in mortality and a 5% increase in MACE (67).
Infarct size by CMR similarly predicts sudden cardiac death (SCD) and arrhythmia after ST segment elevation MI independent of LVEF (68). The authors of that study demonstrated that those with an LVEF of more than 30% with significant scarring (>5% of LV mass) had a similar risk of SCD and appropriate implantable cardiac defibrillator (ICD) discharge than a cohort with LVEF <30%, whilst those with LVEF >30% and minimal or no scarring had a more favourable prognosis, suggesting scar could be potentially used in risk stratification models for ICD implantation in the future.
Furthermore, after STEMI the presence of MO is recognised as an independent marker of subsequent adverse LV remodelling and a strong predictor of MACE (69). Whilst recent studies have shown the presence of IMH identified by CMR is associated with other markers of adverse outcome such as larger infarct size, greater MO and lower LVEF, it may also be a strong independent marker of adverse remodelling and 6 months MACE (52,70,71). There is however a paucity of long term CMR outcome data from large prospective, randomized controlled trials and prognostic information from CE-MARC and MR-IMPACT II trials are eagerly awaited.
Conclusions
CMR is a well-established non-invasive imaging technique with major applications in the evaluation of patients with CHD. In a single imaging session, CMR can assess cardiac anatomy, function, myocardial perfusion and tissue viability, without exposure to ionising radiation. Its use in both stable CHD and ACS is supported by a strong and rapidly expanding evidence-base. However the real challenge for any cardiovascular imaging modality is how it can change patient management and impact upon clinical outcomes. In this regard major on-going clinical trials are likely to raise the prominence of CMR in international guidelines and routine cardiological practice.
Acknowledgements
Funding: JPG and SP received a research grant from Philips Healthcare. SP is funded by a British Heart Foundation Senior Fellowship (FS/10/62/28409).
Disclosure: The authors declare no conflict of interest.
References
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933-44. [PubMed]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:e6-e245. [PubMed]
- British Heart Foundation Statistics Database. British Heart Foundation; Available online: http://www.bhf.org.uk/. Accessed 14th October 2013.
- Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471. [PubMed]
- Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [PubMed]
- Assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (CG95). London: National Institute for Health and Care Excellence, 2010.
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. [PubMed]
- Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012;59:1719-28. [PubMed]
- Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453-60. [PubMed]
- Greenwood JP, Maredia N, Radjenovic A, Brown JM, Nixon J, Farrin AJ, Dickinson C, Younger JF, Ridgway JP, Sculpher M, Ball SG, Plein S. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials 2009;10:62. [PubMed]
- Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, Bijsterveld P, Dickinson CJ, Ball SG, Plein S. Comparison of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Women with Suspected Coronary Artery Disease from the CE-MARC Trial. Circulation 2014;129:1129-38. [PubMed]
- Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Luchner A, Strohm O, Ahlstrom H, Dill T, Hoebel N, Simor T. MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 2013;34:775-81. [PubMed]
- Klein C, Gebker R, Kokocinski T, Dreysse S, Schnackenburg B, Fleck E, Nagel E. Combined magnetic resonance coronary artery imaging, myocardial perfusion and late gadolinium enhancement in patients with suspected coronary artery disease. J Cardiovasc Magn Reson 2008;10:45. [PubMed]
- Bettencourt N, Ferreira N, Chiribiri A, Schuster A, Sampaio F, Santos L, Melica B, Rodrigues A, Braga P, Teixeira M, Leite-Moreira A, Silva-Cardoso J, Portugal P, Gama V, Nagel E. Additive value of magnetic resonance coronary angiography in a comprehensive cardiac magnetic resonance stress-rest protocol for detection of functionally significant coronary artery disease: a pilot study. Circ Cardiovasc Imaging 2013;6:730-8. [PubMed]
- Bonow RO. What’s past is prologue: advances in cardiovascular imaging. Lancet 2012;379:393-5. [PubMed]
- Walker S, Girardin F, McKenna C, Ball SG, Nixon J, Plein S, Greenwood JP, Sculpher M. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart 2013;99:873-81. [PubMed]
- Boldt J, Leber AW, Bonaventura K, Sohns C, Stula M, Huppertz A, Haverkamp W, Dorenkamp M. Cost-effectiveness of cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary artery disease in Germany. J Cardiovasc Magn Reson 2013;15:30. [PubMed]
- Moschetti K, Muzzarelli S, Pinget C, Wagner A, Pilz G, Wasserfallen JB, Schulz-Menger J, Nothnagel D, Dill T, Frank H, Lombardi M, Bruder O, Mahrholdt H, Schwitter J. Cost evaluation of cardiovascular magnetic resonance versus coronary angiography for the diagnostic work-up of coronary artery disease: application of the European Cardiovascular Magnetic Resonance registry data to the German, United Kingdom, Swiss, and United States health care systems. J Cardiovasc Magn Reson 2012;14:35. [PubMed]
- Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol 2007;50:1343-53. [PubMed]
- Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, Ellmer A, Dreysse S, Fleck E. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 1999;99:763-70. [PubMed]
- Korosoglou G, Elhmidi Y, Steen H, Schellberg D, Riedle N, Ahrens J, Lehrke S, Merten C, Lossnitzer D, Radeleff J, Zugck C, Giannitsis E, Katus HA. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1,493 consecutive patients: assessment of myocardial wall motion and perfusion. J Am Coll Cardiol 2010;56:1225-34. [PubMed]
- Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation 2002;106:2328-33. [PubMed]
- Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation 2007;115:1769-76. [PubMed]
- Poldermans D, Fioretti PM, Boersma E, Bax JJ, Thomson IR, Roelandt JR, Simoons ML. Long-term prognostic value of dobutamine-atropine stress echocardiography in 1737 patients with known or suspected coronary artery disease: A single-center experience. Circulation 1999;99:757-62. [PubMed]
- Wahl A, Paetsch I, Gollesch A, Roethemeyer S, Foell D, Gebker R, Langreck H, Klein C, Fleck E, Nagel E. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: experience in 1,000 consecutive cases. Eur Heart J 2004;25:1230-6. [PubMed]
- Kuijpers D, Janssen CH, van Dijkman PR, Oudkerk M. Dobutamine stress MRI. Part I. Safety and feasibility of dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol 2004;14:1823-8. [PubMed]
- Motwani M, Maredia N, Fairbairn TA, Kozerke S, Radjenovic A, Greenwood JP, Plein S. High-resolution versus standard-resolution cardiovascular MR myocardial perfusion imaging for the detection of coronary artery disease. Circ Cardiovasc Imaging 2012;5:306-13. [PubMed]
- Cheng AS, Pegg TJ, Karamitsos TD, Searle N, Jerosch-Herold M, Choudhury RP, Banning AP, Neubauer S, Robson MD, Selvanayagam JB. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol 2007;49:2440-9. [PubMed]
- Motwani M, Jogiya R, Kozerke S, Greenwood JP, Plein S. Advanced cardiovascular magnetic resonance myocardial perfusion imaging: high-spatial resolution versus 3-dimensional whole-heart coverage. Circ Cardiovasc Imaging 2013;6:339-48. [PubMed]
- Plein S, Schwitter J, Suerder D, Greenwood JP, Boesiger P, Kozerke S. k-Space and time sensitivity encoding-accelerated myocardial perfusion MR imaging at 3.0 T: comparison with 1.5 T. Radiology 2008;249:493-500. [PubMed]
- Manka R, Paetsch I, Kozerke S, Moccetti M, Hoffmann R, Schroeder J, Reith S, Schnackenburg B, Gaemperli O, Wissmann L, Wyss CA, Kaufmann PA, Corti R, Boesiger P, Marx N, Lüscher TF, Jahnke C. Whole-heart dynamic three-dimensional magnetic resonance perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve: determination of volumetric myocardial ischaemic burden and coronary lesion location. Eur Heart J 2012;33:2016-24. [PubMed]
- Jogiya R, Kozerke S, Morton G, De Silva K, Redwood S, Perera D, Nagel E, Plein S. Validation of dynamic 3-dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol 2012;60:756-65. [PubMed]
- Hussain ST, Paul M, Plein S, McCann GP, Shah AM, Marber MS, Chiribiri A, Morton G, Redwood S, MacCarthy P, Schuster A, Ishida M, Westwood MA, Perera D, Nagel E. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J Cardiovasc Magn Reson 2012;14:65. [PubMed]
- Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA; American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Nuclear Cardiology; American College of Radiology; American Heart Association; American Society of Echocardiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; Society of Nuclear Medicine. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol 2009;53:2201-29. [PubMed]
- Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004;44:2173-81. [PubMed]
- Greenwood JP, Younger JF, Ridgway JP, Sivananthan MU, Ball SG, Plein S. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging vs exercise tolerance testing early after acute ST elevation myocardial infarction. Heart 2007;93:1363-8. [PubMed]
- Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113:1865-70. [PubMed]
- Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51:1581-7. [PubMed]
- Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 2004;109:2411-6. [PubMed]
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007;93:1547-51. [PubMed]
- Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 1996;94:3318-26. [PubMed]
- Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, Waltenberger J, ten Berg JM, Doevendans PA, Aengevaeren WR, Zwaginga JJ, Biemond BJ, van Rossum AC, Piek JJ, Zijlstra F. HEBE Investigators. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J 2011;32:1736-47. [PubMed]
- Mather AN, Lockie T, Nagel E, Marber M, Perera D, Redwood S, Radjenovic A, Saha A, Greenwood JP, Plein S. Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2009;11:33. [PubMed]
- Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation 2000;101:2734-41. [PubMed]
- Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765-72. [PubMed]
- Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 2005;26:549-57. [PubMed]
- Choi CJ, Haji-Momenian S, Dimaria JM, Epstein FH, Bove CM, Rogers WJ, Kramer CM. Infarct involution and improved function during healing of acute myocardial infarction: the role of microvascular obstruction. J Cardiovasc Magn Reson 2004;6:917-25. [PubMed]
- Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 2008;52:181-9. [PubMed]
- de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 2010;31:2660-8. [PubMed]
- Basso C, Corbetti F, Silva C, Abudureheman A, Lacognata C, Cacciavillani L, Tarantini G, Marra MP, Ramondo A, Thiene G, Iliceto S. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol 2007;100:1322-7. [PubMed]
- van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, Duncker DJ, Wielopolski PA. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J 2006;27:1620-6. [PubMed]
- Mather AN, Fairbairn TA, Ball SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart 2011;97:453-9. [PubMed]
- Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, Bogaert J. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002;106:2873-6. [PubMed]
- Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445-53. [PubMed]
- Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging 2012;5:494-508. [PubMed]
- Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation 2001;104:1101-7. [PubMed]
- Shah DJ, Kim HW, James O, Parker M, Wu E, Bonow RO, Judd RM, Kim RJ. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA 2013;309:909-18. [PubMed]
- Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890-6. [PubMed]
- Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, Erdmann E. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol 1998;31:1040-8. [PubMed]
- Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:826-38. [PubMed]
- Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol 2007;49:227-37. [PubMed]
- Buckert D, Dewes P, Walcher T, Rottbauer W, Bernhardt P. Intermediate-term prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: a prospective follow-up study in a consecutive patient population. JACC Cardiovasc Imaging 2013;6:56-63. [PubMed]
- Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006;113:2733-43. [PubMed]
- Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol 2012;60:408-20. [PubMed]
- Kwon DH, Halley CM, Carrigan TP, Zysek V, Popovic ZB, Setser R, Schoenhagen P, Starling RC, Flamm SD, Desai MY. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging 2009;2:34-44. [PubMed]
- Yokota H, Heidary S, Katikireddy CK, Nguyen P, Pauly JM, McConnell MV, Yang PC. Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson 2008;10:17. [PubMed]
- Zemrak F, Petersen SE. Late gadolinium enhancement CMR predicts adverse cardiovascular outcomes and mortality in patients with coronary artery disease: systematic review and meta-analysis. Prog Cardiovasc Dis 2011;54:215-29. [PubMed]
- Izquierdo M, Ruiz-Granell R, Bonanad C, Chaustre F, Gomez C, Ferrero A, Lopez-Lereu P, Monmeneu JV, Nuñez J, Chorro FJ, Bodi V. Value of early cardiovascular magnetic resonance for the prediction of adverse arrhythmic cardiac events after a first noncomplicated ST-segment-elevation myocardial infarction. Circulation 2013;6:755-61. [PubMed]
- Cochet AA, Lorgis L, Lalande A, Zeller M, Beer JC, Walker PM, Touzery C, Wolf JE, Brunotte F, Cottin Y. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol 2009;19:2117-26. [PubMed]
- Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 2009;30:1440-9. [PubMed]
- Eitel I, Kubusch K, Strohm O, Desch S, Mikami Y, de Waha S, Gutberlet M, Schuler G, Friedrich MG, Thiele H. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging 2011;4:354-62. [PubMed]


