CT-angiography protocol with low dose radiation and low volume contrast medium for non-cardiac chest pain
Introduction
Chest pain is one of the most common chief complaints for patients who present to the emergency department. The clinical presentation of chest pain can be highly variable and encompasses a wide spectrum of non-vascular or vascular factors such as pneumonia, pleural or chest wall problems, aortic dissection, pulmonary embolism and coronary artery disease (1,2). In patients with chest pain, scanning computed tomography (CT) protocols vary to facilitate visualization of various major vascular components in the chest, such as pulmonary arteries, aorta or coronary arteries and their branches. Multiple detector-row computed tomography angiography (MDCT-A) technology has facilitated imaging of coronary arteries along with the entire thorax, in a single breath-hold.
CT angiography (CT-A) is able to simultaneously evaluate for multiple potentially life-threatening conditions, including acute coronary syndrome, aortic dissection, and pulmonary thromboembolism (3). With increasing concern regarding the radiation associated with medical imaging, scanning protocols have been developed to minimize the radiation doses (4). In patients with renal failure and co-morbid factors, MDCT-A has another disadvantage known as contrast-induced nephropathy (CIN) (5). CIN is described as acute renal failure occurring within 48 h. of exposure to intravascular radiographic contrast agent that is not attributable to other possible causes. The incidence of CIN as a complication of radiological studies varies significantly, depending on the technique used and other variables such as the type of radiological procedure performed, the dose and type of contrast media administered the differing patient groups in terms of number and type of potential risk factors.
Although numerous dose-saving techniques have been described for CT-A, the applicability of these techniques combined with contrast media dose-saving technique has been infrequently described in published studies (6-8). The purpose of the present study was to evaluate the diagnostic quality of MDCT-A protocol using a new dose-saving technique for evaluation of non-cardiac chest pain.
Materials and methods
Study group
A total of 45 consecutive patients (24 women, 21 men) with a mean age of 47 (range, 34-79) years, requiring contrast-enhanced chest CT were examined. The patients were assigned to the protocol, with 80 kilovolt (peak) (kV[p]) and 150 effective milliampere-second (eff mA-s). Except the amount of contrast material, type of contrast material (Iomeprol, Iomeron 400®, Bracco spa, Milano, Italy), contrast administering technique, radiation exposure, and all other technical parameters are similar for all patients in the study group.
In each patient, bolus tracking was monitored with a low-dose automatic timing device (Care Bolus; Siemens Medical Solutions) to optimize the delay time from the start of injection to the start of scanning at the level of the ascending aorta. The aim of contrast injection is to obtain a continuous and enough long phase of aortic and pulmonary arterial opacification during which data can be acquired in both vascular structures. Pulmonary arterial and aortic enhancement is related to the flow of opacified blood transition through the pulmonary circulation and then flowing into the aorta. Generally, optimal imaging of the thoracic aorta and is obtained with scanning delays of 20-30 s (9). In our study group, 40 mL of low osmolar contrast material was administered at 3.0 mL/s. After the completion of 30 mL of contrast administration within 10 s. as measured by a stopwatch, the scanning was started. However, because of a 5 s-delay, the scanning was actually started at 15 s. Saline flush was used after contrast administration.
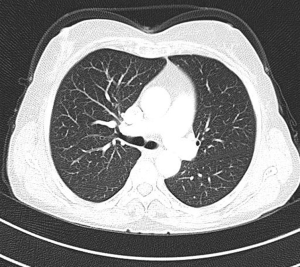
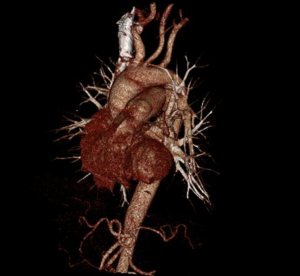
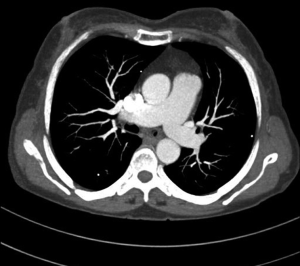
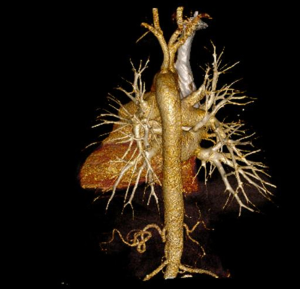
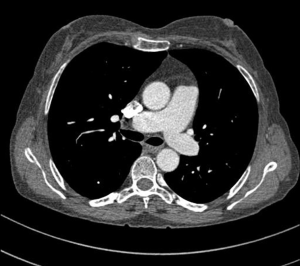
MDCT-A examinations were performed with a 128-section multidetector CT (Siemens Definition AS +, Siemens Medical Solutions, Forchheim, Germany). MDCT-A is performed during an inspiratory breath-holding, which should never exceed 25 s. Patients need to be completely informed of the significance of holding their breath to ensure a high-quality imaging; crucially ill patients should be connected to an oxygen mask. Ideal protocols for scanning are obligatory for entire and right assessment of emergent vascular conditions with MDCT, and the intravenous (IV) contrast agent injection protocol must be specific to the vascular region. A craniocaudal scanning direction was chosen. Patients were examined in the supine position with both arms extended above the head. Scan volume ranged from the level of the right diaphragm to a level just above the thoracic inlet. Subsequent reconstructed axial images of 2 mm slice thickness were obtained using a medium-sharp convolution kernel (B20 f) with an image matrix of 512×512 pixels. We used a MDCT-A window setting (width, 400 HU; level, 100 HU) and a lung window setting (width, 1,500 HU; level, –500 HU) for this analysis (10). For all examinations, vascular enhancement was achieved by the same level of main pulmonary artery and ascending aorta use of an IV injection of contrast material via a cubital vein. In the study group, we evaluated the mean CT number (as HU) in the main pulmonary artery and ascending aorta by using a region of interest (ROI) of average (range, 1-1.4 cm2) in the same axial images (Figures 1,2,3). Flow rate was kept constant at 3 mL/s throughout the procedure. In adult patients (weigh range, 60-120 kg), a flow rate of 4 mL/s for a 20 s duration usually opacifies the thoracic aorta at a level greater than 200 HU, and this is generally suitable for MDCT-A and postprocessing evaluation (7). A workstation (Leonardo, Siemens AG, Erlanger, Germany) was used for interpretation of the image data. Sagittal and coronal MPR images were obtained to evaluate both the pulmonary arteries and the aorta with supradiaphragmatic branches simultaneously (Figures 4,5,6). All pre- and post-processing procedures were performed by two experienced radiologist who had over 10-year experience.

Patients with irregular heart rates were included in the study. No additional premedication and ECG-gated imaging for heart rate control was used. Exclusion criteria for the study were nephropathy (described as a serum creatinine level greater than 1.5 mg/dL), BMI >25, known hypersensitivity to contrast media which iodine containing, inability to provide informed consent, extreme claustrophobia, pregnancy or unknown pregnancy status, age younger than 18 years, clinical instability as deemed by the attending physician, including patients with complete heart block; second-degree atrioventricular block and CT imaging or contrast material administration within the past 48 hours.
The institutional review board of our medical center approved this prospective study. Written informed consent was obtained from all patients before the chest CT scan. No patient preparation was required for the study protocol. Procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Results
A total of 45 consecutive patients (24 women, 21 men) with a mean age of 47 (range, 34-79) years, were referred to radiology department for MDCT-A as clinically suspected vascular causes of non-cardiac chest pain from March 2011 to March 2012. The study group consisted of 24 men and 21 women with an age range of 34 to 79 years (mean 47 years). In the study group, four patients with pulmonary embolism, four with pleural effusion, two with ascending aortic aneurysm and eight patients with pneumonic consolidation were detected. The mean segment attenuation of the pulmonary truncus and ascendant thoracic aorta were above the selected diagnostic threshold of 180 HU. In the study group, the mean attenuation of all the pulmonary truncus and ascending aortic locations was considered 264±44 and 249±51 HU, respectively. A total of 22 patients had an average attenuation below 210 HU in the main pulmonary artery. We provided adequate enhancement in both vascular systems in whole of the cases. All the patients were followed-up by clinicians. The mean effective radiation dose was 0.83 mSv for MDCT-A.
Discussion
The correct identification of patients who have a life-threatening cause of chest pain enables early intervention, preservation of viable myocardium, and improved outcomes. Furthermore, prevention of unnecessary hospital admissions for chest pain limits the associated morbidities and costs of hospitalization. This makes chest pain one of the most challenging clinical presentations facing the emergency department physician. Prior to the introduction of MDCT scanners, electron beam computed tomography—with better temporal but inferior spatial resolution as compared to MDCT—had been proposed as a tool that could be used in the evaluation of patients in the emergency department with chest pain. Recently, MDCT-A has become an essential diagnostic imaging method for detection of pulmonary embolism and aortic disorders which causes of chest pain (1-3,5,8). Although MDCT-A has evolved into an invaluable diagnostic tool, attention has been focused on the issues of high dose radiation exposure and nephropathy induced by contrast medium.
Typical protocols require multiple image acquisitions to obtain the pulmonary arterial and aortic phase images which increases the patient’s radiation exposure (1,2). Besides, patient must be administered contrast media at high concentration, so, the risk of nephropathy significantly increases. Thus, in this defined patient groups are composed of prominent limitations for diagnostic imaging, particularly (2,5,7,9).
In recent years, there has been a significant amount of media attention on the appropriate and inappropriate radiation exposure from medical diagnostic imaging procedures and the potential risk of cancer (11,12). This has caused not only great concern among patients but also medical community as well as stimulated the development of systems and acquisition strategies to reduce radiation exposure. There are several practical means for reducing the CT radiation dose. The parameters that affect the CT radiation dose include tube current and voltage, scanning modes, and scan length (13). Several studies on the chest CT have suggested that it is possible to reduce tube current without significantly affecting the image quality (5-9). In general, the dose and radiation exposure of the CT varies with the square of the kilovoltage in the setting of a constant tube current (4). Therefore, reducing the tube voltage has a greater effect on the reduction of the radiation exposure than does reducing the tube current. Careful selection of CT scanning protocols is needed to keep the radiation exposure ‘as low as reasonably achievable’ (ALARA) (3,14). ALARA principle emphasizes the need to perform justifiable CT scans with the minimum radiation dose necessary to meet clinical and diagnostic objectives. We significantly reduced the radiation exposure in the present study and the mean radiation dose was 0.83 mSv. Thus, we scanned and evaluated rapidly MDCT-A especially in emergency room patients.
Many of the studies in literature, amount of contrast material are kept on 100 cc and over, although shorter acquisition time (2,5,7,9). As far as we know in the literature, most of authors accepted concentrations for adults with normal renal function tolerate at least 100 mL of 300 mgI/mL contrast medium very well; for children, 2-3 mL of 300 mgI/mL per kilogram typically is injected (7). Frauenfelder et al. administered 150 mL of a contrast material with 4 slice CT scanner in adult patients (2). Türkvatan et al. performed their examinations with a 16-row MDCT after an injection of 1.5-2 mL/kg contrast agent (15). In the present study, we have significantly reduced to contrast-induced nephropathy and the possibility of allergic reactions theoretically by reducing the amount of contrast material in our trial.
In the current literature, virtual 70-keV monoenergetic CTPA image datasets were found to significantly increase vessel attenuation and contrast-to-noise ratio of dual energy CT pulmonary angiography (16). These findings remind that low-KeV monoenergetic reconstructions may lead to a decrease in the amount of iodinated contrast for adequate image quality. In another recent publication, 40 mL contrast was used at 80 kV and automated current adjustment of 50-300 mA was made (17). It was noted that 80-kV pulmonary CTA of lean patients provided higher intravascular enhancement with 40 mL of iodixanol (320 mgI/mL) than with the same volume of iomeprol (400 mgI/mL), with good vessel conspicuity down to the subsegmental level. Our results are consistent with these data and we suggest that radiation exposure can be reduced significantly with dose saving techniques in visualization of pulmonary artery and aorta.
Several limitations of this study should be mentioned: First, the present study was performed on a relatively small study group that may not be enough to represent the whole community. Second, ECG monitoring and coronary arteries imaging were not obtained. However, due to this limitation, our technique was performed all patient groups including patients with heart problems, such as arrhythmia etc. An additional consideration is the relatively low sensitivity of this exam to diagnose chest pain due to musculoskeletal problems and gastroesophageal reflux disease. Thus, although many life-threatening diseases could be diagnosed or excluded, not every cause of chest pain can be diagnosed with this protocol. Moreover, such a low Kvp protocol may not be sufficient in obese patients and higher Kvps may be required. Given that the average attenuation of at least 200-210 HU is required and the average attenuation of emboli is about 80-90 HU (18), adequate opacification in segmental pulmonary artery branches to detect all emboli may not be fully accomplished.
In conclusion, pulmonary artery and the aorta scanning simultaneously was significantly reduced radiation exposure with the mentioned dose saving technique. Additionally, injection of low volume (40 cc) contrast material may reduce the risk of contrast induced nephropathy, therefore, facilitate the diagnostic approach. This technique can be applied to all cases particularly contrast agent-risk group due to its similar diagnostic quality with a low dose and volume as well as high levels of arteriovenous enhancement simultaneously.
Acknowledgements
The authors are grateful to Ali Ozan Malkoc (aliozanmalkoc@msn.com) and Celal Uzungunay (celaluzungunay@hotmail.com) for their assistance in data collection.
Disclosure: The authors declare no conflict of interest.
References
- Salvolini L, Renda P, Fiore D, Scaglione M, Piccoli G, Giovagnoni A. Acute aortic syndromes: Role of multi-detector row CT. Eur J Radiol 2008;65:350-8. [PubMed]
- Frauenfelder T, Wildermuth S, Marincek B, Boehm T. Nontraumatic emergent abdominal vascular conditions: advantages of multi-detector row CT and three-dimensional imaging. Radiographics 2004;24:481-96. [PubMed]
- Gallagher MJ, Raff GL. Use of multislice CT for the evaluation of emergency room patients with chest pain: the so-called “triple rule-out”. Catheter Cardiovasc Interv 2008;71:92-9. [PubMed]
- Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23. [PubMed]
- Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 1989;320:143-9. [PubMed]
- Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, Alkadhi H, Wildermuth S. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol 2008;18:1809-17. [PubMed]
- Einstein AJ, Wolff SD, Manheimer ED, Thompson J, Terry S, Uretsky S, Pilip A, Peters MR. Comparison of image quality and radiation dose of coronary computed tomographic angiography between conventional helical scanning and a strategy incorporating sequential scanning. Am J Cardiol 2009;104:1343-50. [PubMed]
- Bischoff B, Hein F, Meyer T, Krebs M, Hadamitzky M, Martinoff S, Schömig A, Hausleiter J. Comparison of sequential and helical scanning for radiation dose and image quality: results of the Prospective Multicenter Study on Radiation Dose Estimates of Cardiac CT Angiography (PROTECTION) I Study. AJR Am J Roentgenol 2010;194:1495-9. [PubMed]
- Rubin GD. Angiography of the aorta and its branches. In: Fishman EK, Jeffrey JR EB. eds. Multidetector CT. Philadelphia: Lippincott Williams & Wilkins, 2004;395-441.
- Higgins CB. Modern imaging of the acute aortic syndrome. Am J Med 2004;116:134. [PubMed]
- Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23. [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [PubMed]
- Lee HY, Yoo SM, White CS. Coronary CT angiography in emergency department patients with acute chest pain: triple rule-out protocol versus dedicated coronary CT angiography. Int J Cardiovasc Imaging 2009;25:319-26. [PubMed]
- Mettler FA Jr, Thomadsen BR, Bhargavan M, Gilley DB, Gray JE, Lipoti JA, McCrohan J, Yoshizumi TT, Mahesh M. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys 2008;95:502-7. [PubMed]
- Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol 2009;10:176-84. [PubMed]
- Apfaltrer P, Sudarski S, Schneider D, Nance JW Jr, Haubenreisser H, Fink C, Schoenberg SO, Henzler T. Value of monoenergetic low-kV dual energy CT datasets for improved image quality of CT pulmonary angiography. Eur J Radiol 2014;83:322-328. [PubMed]
- Faggioni L, Neri E, Sbragia P, Pascale R, D’Errico L, Caramella D, Bartolozzi C. 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL)and iomeprol (400 mg I/mL). AJR Am J Roentgenol 2012;199:1220-5. [PubMed]
- Wittram C, Maher MM, Halpern EF, Shepard JA. Attenuation of acute and chronic pulmonary emboli. Radiology 2005;235:1050-4. [PubMed]