Cardiovascular magnetic resonance T2* for tissue iron assessment in the heart
Background
Iron deposition in the heart due to repeated blood transfusion can cause a progressive cardiomyopathy, resulting in cardiovascular complications which remain the cause of the majority of deaths in patients with thalassemia major (TM) (1-3). The clinical manifestations of myocardial siderosis often occur late and, once heart failure develops, the outcome is usually poor despite intensive chelation (4). This iron-induced cardiomyopathy, however, can be reversed if intensive chelation is instituted at an early stage. Myocardial iron measurement can therefore play an important role in assessing the prevalence of myocardial siderosis (5), predicting the risk of cardiac complications (6), and the tailoring of cardiac optimized iron-chelating treatment (7-9).
Although serum ferritin is clinically used to estimate body iron stores, it reflects approximately 1% of the total iron storage pool and its measurement can be confounded by a number of conditions such as inflammation, abnormal liver function, and ascorbate deficiency (10). In contrast to serum ferritin, liver iron can serve as a better indicator of whole body iron; however, liver iron does not reflect heart iron. Significant cardiac iron overload and toxicity can occur despite low liver iron concentrations (11). The measurement of cardiac iron posed a great challenge to the society. Not only is endomyocardial biopsy highly risky, but the measurement taken is also potentially inaccurate due to the small size of the sample obtained and heterogeneous deposition of cardiac iron. The introduction of cardiovascular magnetic resonance (CMR) provided a reliable measure of tissue iron and revolutionized our understanding and management of iron induced cardiomyopathy.
Development of CMR T2* for tissue iron assessment
Tissue iron can be detected indirectly by measuring the relaxation times of hydrogen nuclei affected by ferritin and hemosiderin iron. The presence of this iron results in the shortening of proton relaxation times, particularly T2, an effect termed susceptibility-induced relaxation (12). Early CMR techniques employing T2 measurements by the spin-echo (SE) sequence (or its variant) were useful in quantifying liver iron as compared with liver biopsy (13,14), but unsatisfactory for measurement of iron in the heart due to hardware constraints, flow, motion and noise (15,16).
Knowing the limitations of the myocardial T2 measurement sequence for myocardial iron assessment, Anderson et al. investigated an alternative T2* technique using a gradient-echo sequence with multiple breath-hold for the same purpose (17). This has been demonstrated to be reliable for detecting and evaluating the extent of cardiac iron deposition early. Clinically important iron loading is defined by T2* values of less than 20 ms (17), and severe cardiac iron loading is considered present if cardiac T2* is less than 10 ms (6). A single breath-hold multi-echo technique was subsequently developed, which has the combined advantages of sensitivity, ease of registration, and improved rapidity (18). Table 1 shows typical imaging parameters for the breath-hold T2* using 1.5T scanner. It should be noted that these parameters (particularly TE and TR) may vary from scanner to scanner, but if kept similar to those described in Table 1, the small variations are not expected to affect the T2* measurement in any significant way.
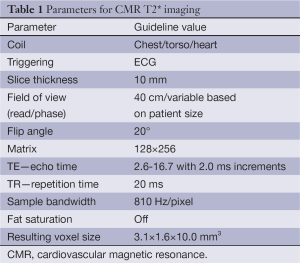
Full table
Despite the success of the breath-hold T2* technique, myocardial T2* measurements are subject to artifacts generated from myocardial motion, and those from blood such as ghosting artifacts and partial volume effects (18). Images acquired at late diastole in bright-blood imaging appear more suitable for T2* measurement as there are few motion artifacts in this cardiac phase, however, the blood signal may still spoil the myocardium and blur its borders. To address these issues, a black-blood sequence using a double inversion recovery (DIR) pulse (19) to null the signal from blood and with acquisition of the multiecho T2* images in late diastole, when cardiac motion is negligible, was developed (20). Compared with bright-blood images, the black-blood images have superior contrast and improve myocardial border definition. The black-blood technique produced less bias and reduced interobserver variability. These initial findings were subsequently confirmed on a large patient population (21).
T2* measurement techniques
This T2* technique was originally developed with the aim of minimizing imaging artifacts, e.g., flow compensation was used and the respiration motioned was suppressed by breath-holding. For the measurement of myocardial T2* in vivo, a mid-ventricular short axis slice is acquired and a homogeneous region of interest (ROI) is defined encompassing both epicardial and endocardial regions as iron is preferentially laid down in the epicardium compared with the endocardium. The analysis is restricted to the septum to avoid susceptibility artifacts which arise from the anterior and posterior cardiac vessels veins and the lung (Figure 1). In addition, T2* in the septum has proven to be a good indicator of the global iron in the heart (22). Aiming at addressing heterogeneity in iron distribution in the myocardium, a multi-slice T2* acquisition has also been proposed, but it is time-consuming and the analysis is confounded by inclusion of susceptibility artifacts. To date, no significant clinical advantage has been demonstrated using the multi-slice technique. The single-slice T2* technique remains the preferred protocol in the practice (23).

The signal intensity of the ROI is measured for each of the T2* images, and the data is plotted against the echo time to form an exponential decay curve. Initially, the decay rate T2* was derived by fitting a mono-exponential trend line to the exponential decay curve (17); subsequent studies suggested that a more complex nonlinear algorithm should be used for improved curve fitting and more accurate T2* measurement (24,25). In the presence of severe myocardial iron overload, a rapid decay in myocardial signal intensity can lead to a plateau in the later echo time images. In this scenario, the truncation model (24-26) can provide more accurate and reproducible measurement than the alternative offset model (27). This particular problem is less pronounced in T2* measurement using the black-blood technique as the main source of error is largely removed (Figure 2).

Reproducibility and transferability of CMR T2*
In order for the T2* sequence to be suitable for the clinical assessment of tissue iron, good reproducibility of the technique is necessary and both methods have demonstrated good interstudy reproducibility where T2* has been measured on two separate occasions (17,18). In addition, for maximal healthcare impact, transferability between scanners of different manufacture and between site must be established. It was initially demonstrated that the multiple breath-hold technique was transferable between two scanners in the same site (28) with good reproducibility. Supported by Thalassemia International Federation (TIF), the initial finding was further confirmed through a multicenter study, in which patient scans were performed locally in six different countries and subsequently rescanned in the standardized center in London within one month (29). The single breath-hold T2* technique was also validated but the initial attempt was limited with only three scanners in Italy and UK and on a small patient population (30). With support and funding from National Institutes of Health (NIH), a study assessing the reproducibility and transferability of the breath-hold T2* technique was conducted in five international centers using standardized acquisition and analysis techniques (31).
Current status and future directions
Unlike T2*, both T2 and T1 are not affected by extrinsic magnetic field inhomogeneity. There have been attempts to develop T2 and T1 techniques and the interest in a comparison of these relaxation parameters to identify if additional useful information can be gleaned. The attempt for a single breath-hold T2 technique for tissue iron assessment succeeded with technical advances (32). The investigation of the relationship between myocardial T2 and T2* measurements in a substantial patient population was conducted (33). The pilot data demonstrated that iron deposition is the dominant factor in determining T2* and T2 relaxation of the myocardium, and that both T2* and T2 can provide noninvasive and reproducible measures for myocardial iron assessment in transfusion dependent patients. With the recent development of the modified Look-Locker Inversion recovery (MOLLI) sequence (34), T1 changes in response to myocardial iron deposition was investigated and the study further demonstrated that there is a linear correlation between T1 and T2 in the human heart, and that T1 can also be used to assess myocardial iron (35). The exact manner in which tissue iron affects CMR relaxometry remains unclear. A more comprehensive and detailed study investigating the relationship between iron deposition and T1, T2, and T2* may shed light on this fundamental issue. From a clinical perspective, it would be useful if CMR relaxometry could be used to distinguish between different forms of storage iron. A novel method has been developed to separate the two principal forms of tissue storage iron: ferritin and hemosiderin (36); further studies are needed to demonstrate its clinical benefit. Studies integrating multiple CMR relaxation parameters can offer potential to improve our undemanding in this regard.
T2* value is dependent on field strength. To date, nearly all validation and calibration work relating to T2* and iron assessment has been conducted at 1.5T. With the growing popularity of 3T magnets, a calibration of T2* between 3T and 1.5T is needed in order to establish the ranges for normal, mild, and severe iron overload to help diagnosis, treatment and patient follow-up.
Tissue iron overload is a global disease, and it is important to expand patient access to cardiac iron assessment. Ideally therefore any such measurement needs to be simple, robust and preferably automated to ensure accurate and reproducible measurements. Currently, T2* analysis is based on manual delineation of the septum, which is time-consuming and introduces human error. A fully automated T2* analysis software integrated with a standardized acquisition protocol is therefore crucial to improve global healthcare.
Conclusions
After a decade of efforts, CMR T2* is currently recognized as the method of choice for the assessment of tissue iron. This T2* technique has been validated in more than 100 international centers by different research groups, and presents itself as one of the most successful examples demonstrating the ability of imaging to alter patient outcome. With the introduction of T2*, advancement of new chelation drugs, and personalized patient management, the mortality rate attributed to tissue iron overload has been decreasing significantly worldwide. Further development is required to improve patient access to reliable T2* measurement.
Disclosure: The author declares no conflict of interest.
References
- Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:42. [PubMed]
- Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, Ghilardi R, Piga A, Romeo MA, Zhao H, Cnaan A. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood 2006;107:3733-7. [PubMed]
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 1994;331:574-8. [PubMed]
- Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077-84. [PubMed]
- Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, Nair SV, Anderson LJ, Walker JM, Pennell DJ. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson 2006;8:543-7. [PubMed]
- Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ, Pennell DJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009;120:1961-8. [PubMed]
- Pennell DJ. T2* magnetic resonance and myocardial iron in thalassemia. Ann N Y Acad Sci 2005;1054:373-8. [PubMed]
- Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA, Wonke B, Galanello R. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006;107:3738-44. [PubMed]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007;115:1876-84. [PubMed]
- Brittenham GM, Cohen AR, McLaren CE, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol 1993;42:81-5. [PubMed]
- Anderson LJ, Westwood MA, Prescott E, Walker JM, Pennell DJ, Wonke B. Development of thalassaemic iron overload cardiomyopathy despite low liver iron levels and meticulous compliance to desferrioxamine. Acta Haematol 2006;115:106-8. [PubMed]
- Gillis P, Roch A, Brooks RA. Corrected equations for susceptibility-induced T2-shortening. J Magn Reson 1999;137:402-7. [PubMed]
- Kaltwasser JP, Gottschalk R, Schalk KP, Hartl W. Non-invasive quantitation of liver iron-overload by magnetic resonance imaging. Br J Haematol 1990;74:360-3. [PubMed]
- Gomori JM, Horev G, Tamary H, Zandback J, Kornreich L, Zaizov R, Freud E, Krief O, Ben-Meir J, Rotem H. Hepatic iron overload: quantitative MR imaging. Radiology 1991;179:367-9. [PubMed]
- Mavrogeni SI, Gotsis ED, Markussis V, Tsekos N, Politis C, Vretou E, Kermastinos D. T2 relaxation time study of iron overload in b-thalassemia. MAGMA 1998;6:7-12. [PubMed]
- Papanikolaou N, Ghiatas A, Kattamis A, Ladis C, Kritikos N, Kattamis C. Non-invasive myocardial iron assessment in thalassaemic patients. T2 relaxometry and magnetization transfer ratio measurements. Acta Radiol 2000;41:348-51. [PubMed]
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22:2171-9. [PubMed]
- Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging 2003;18:33-9. [PubMed]
- Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996;199:49-57. [PubMed]
- He T, Gatehouse PD, Kirk P, Tanner MA, Smith GC, Keegan J, Mohiaddin RH, Pennell DJ, Firmin DN. Black-blood T2* technique for myocardial iron measurement in thalassemia. J Magn Reson Imaging 2007;25:1205-9. [PubMed]
- Smith GC, Carpenter JP, He T, Alam MH, Firmin DN, Pennell DJ. Value of black blood T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13:21. [PubMed]
- Pepe A, Positano V, Santarelli MF, Sorrentino F, Cracolici E, De Marchi D, Maggio A, Midiri M, Landini L, Lombardi M. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging 2006;23:662-8. [PubMed]
- Baksi AJ, Pennell DJ. T2* imaging of the heart: methods, applications, and outcomes. Top Magn Reson Imaging 2014;23:13-20. [PubMed]
- He T, Gatehouse PD, Kirk P, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T*2 measurement in iron-overloaded thalassemia: an ex vivo study to investigate optimal methods of quantification. Magn Reson Med 2008;60:350-6. [PubMed]
- He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T2* measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magn Reson Med 2008;60:1082-9. [PubMed]
- He T, Zhang J, Carpenter JP, Feng Y, Smith GC, Pennell DJ, Firmin DN. Automated truncation method for myocardial T2* measurement in thalassemia. J Magn Reson Imaging 2013;37:479-83. [PubMed]
- Ghugre NR, Enriquez CM, Coates TD, Nelson MD Jr, Wood JC. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging 2006;23:9-16. [PubMed]
- Westwood MA, Anderson LJ, Firmin DN, Gatehouse PD, Lorenz CH, Wonke B, Pennell DJ. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging 2003;18:616-20. [PubMed]
- Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ. Thalassemia International Federation Heart T2* Investigators. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica 2006;91:1388-91. [PubMed]
- Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, Vasili B, Pennell DJ. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging 2005;21:531-8. [PubMed]
- Kirk P, He T, Anderson LJ, Roughton M, Tanner MA, Lam WW, Au WY, Chu WC, Chan G, Galanello R, Matta G, Fogel M, Cohen AR, Tan RS, Chen K, Ng I, Lai A, Fucharoen S, Laothamata J, Chuncharunee S, Jongjirasiri S, Firmin DN, Smith GC, Pennell DJ. International reproducibility of single breathhold T2* MR for cardiac and liver iron assessment among five thalassemia centers. J Magn Reson Imaging 2010;32:315-9. [PubMed]
- He T, Gatehouse PD, Anderson LJ, Tanner M, Keegan J, Pennell DJ, Firmin DN. Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. J Magn Reson Imaging 2006;24:580-5. [PubMed]
- He T, Smith GC, Gatehouse PD, Mohiaddin RH, Firmin DN, Pennell DJ. On using T2 to assess extrinsic magnetic field inhomogeneity effects on T2* measurements in myocardial siderosis in thalassemia. Magn Reson Med 2009;61:501-6. [PubMed]
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141-6. [PubMed]
- Feng Y, He T, Carpenter JP, Jabbour A, Alam MH, Gatehouse PD, Greiser A, Messroghli D, Firmin DN, Pennell DJ. In vivo comparison of myocardial T1 with T2 and T2* in thalassaemia major. J Magn Reson Imaging 2013;38:588-93. [PubMed]
- Jensen JH, Tang H, Tosti CL, Swaminathan SV, Nunez A, Hultman K, Szulc KU, Wu EX, Kim D, Sheth S, Brown TR, Brittenham GM. Separate MRI quantification of dispersed (ferritin-like) and aggregated (hemosiderin-like) storage iron. Magn Reson Med 2010;63:1201-9. [PubMed]


