Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes
Introduction
Medically refractory temporal lobe epilepsy (TLE) is the most common and most frequently operated intractable form of epilepsy (1,2). Resective temporal lobe surgery is effective for many patients with refractory TLE, providing a greater likelihood of seizure freedom and improved quality of life compared to anti-epileptic drug (AED) treatment for suitable patients (3). However, up to 40% of patients with refractory TLE will continue to experience disabling postoperative seizures 2 years after surgery (4-7), and the proportion continuing to have seizures increases with longer postoperative follow-up (8,9). It is currently unknown why a large subgroup of patients continues to experience postoperative seizures despite surgical intervention. Prior to surgery, the patients who achieve complete postoperative seizure control are typically clinically indistinguishable from patients who continue to experience seizures and there is considerable controversy in the current literature regarding presurgical clinical factors that may help predict outcome. Prognostic stratification of patients according to likely outcome is therefore very difficult based on the manifestation of seizures and natural history of the disorder.
Outcome predictors of TLE surgery have been extensively debated in the literature over the past decades. Frequently reported indicators of optimal surgical outcome are unilateral signs of HS on diagnostic MRI (10-13) and unilateral interictal epileptiform discharges (13-15). Other reported predictors of good outcome are younger age at surgery (16), shorter epilepsy duration (16), history febrile seizures (17,18), epileptiform discharge frequency (19), and absence of generalized seizures (20,21).
It is important to note that these indicators are not consistently observed across studies, even among large well-powered analyses. Furthermore, despite that presentation of HS on MRI is most strongly related to an improved outcome, it does not guarantee an optimal one; at 60 months after surgery, only half of all patients with TLE and HS will experience no postoperative seizures (11). Table 1 provides a summary of findings from a sample of moderate to large studies addressing factors predicting seizure outcome after TLE surgery. It is noteworthy that the results from various studies are partially discordant; some features are considered predictive of seizure freedom after surgery in some studies but not in others. While it is possible that specific features may have not been identified as predictors in some studies due to lack of statistical power, it is also equally possible that some features are not predictors for all patients with TLE; in other words, they may not be generalizable to samples of patients outside the specific studies.
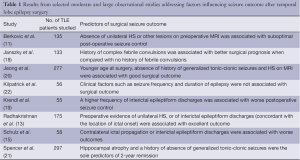
Full table
Overall, the data above demonstrates two important points. First, although unilateral HS appears to be the most reproducible factor leading to optimal outcome, many patients with unilateral HS do not achieve seizure freedom. Second, given the numerous high-quality (single and multi-center) outcome studies performed to date, it is highly unlikely that new discoveries about prognosis can be gained by further studies assessing typical preoperative clinical factors. These points underscore the crucial need for a novel biomarker to predict outcome.
The goal of this paper is to provide a review of the studies that have attempted to determine the preoperative quantitative MRI correlates of postoperative seizure outcome in patients with refractory TLE. The identification of preoperative imaging correlates of persistent postoperative seizures may lead to the development of novel biomarkers of treatment outcome for patients with TLE. We begin by providing an overview of brain alterations in TLE before focusing on studies that have directly examined postoperative outcome, and attempt to resolve whether preoperative quantitative structural alterations have any significance for the prediction of persistent postoperative seizures.
Quantitative MRI in TLE: overview of structural alterations
Volumetric MRI
Quantitative MRI techniques (e.g., hippocampal volumetry) are occasionally used to provide supplementary diagnostic information in context of preoperative evaluation (23,24), and have been widely used to characterize alterations in brain structure in patients with TLE. There are several articles that provided early reports of the reliable detection of hippocampal atrophy (HA) in patients with TLE based on conventional region-of-interest volumetry applied to (typically T1-weighted) MR images as a surrogate marker of HS (25-30). The application of similar MRI methods to other brain structures later revealed extrahippocampal atrophy, particularly of limbic and paralimbic regions (31,32). Atrophy was reported of regions adjacent to and closely connected with the hippocampus, including the amygdala, and entorhinal and perirhinal cortices (33-36), Zand of lateral neocortical temporal lobe regions (37) preferentially, but not exclusively, to the side of seizure onset. Structural alterations were also reported of deep grey matter nuclei known to be important for the modulation, propagation and expression of focal seizures, including the thalamus and striatum (38-40). Whether these changes are the result of recurrent uncontrolled seizures or are pre-existing is of considerable debate (41). Some studies have revealed a significant relationship between the duration of TLE and hippocampal (42-45) and extrahippocampal (39,46) volume, and between estimated number of seizures and volume (47,48), suggesting that the chronicity of the disorder, potentially including the excitotoxic effects of recurrent seizures, has a pathologically degenerating effect on the brain. Other cross-sectional studies reported no relationships between volume and duration of TLE (49,50). Some longitudinal studies have reported subtle progressive volume loss of the presumed epileptogenic hippocampus (51-53) and extrahippocampal cortex (54) across cohorts of patients with TLE, whilst others have not (55). The observed inconsistency in volumetric MRI studies on the progression of brain atrophy and the etiology of HS in refractory TLE mirrors that seen in experimental animal studies of induced epilepsy (56).
One important limitation associated with manual morphometry techniques is with respect to their limited applicability in clinical practice. They require a time-consuming process by a skilled rater that is usually impractical in busy clinical centers. Furthermore, the reliability and reproducibility of the method should be periodically checked since the results can be dependent on the rater, posing significant problems if there is a change in staff (e.g., if the rater who assessed the normative sample of controls is not the same as the rater who tests patients). For these reasons, a clinically useful biomarker should rely on automated, rater independent measures that are biologically substantiated.
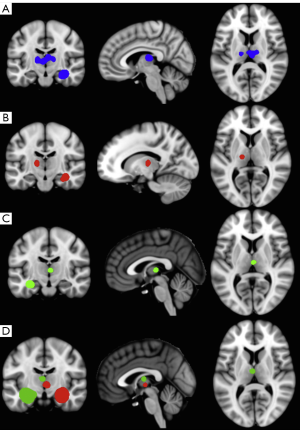
Developments in MRI analysis techniques throughout the late 1990s and 2000s permitted the automated quantitative analysis of brain structure not restricted to one region-of-interest, overcoming the time and resource consuming nature of human volumetric measurements. The most widely applied automated MRI analysis technique applied to refractory TLE is voxel-based morphometry (VBM) (57). To our knowledge, there are five review articles currently published on the application of VBM studies to study brain alterations in refractory TLE (58-62), and the reader is referred to these papers for a detailed review of studies. Generally, VBM studies have revealed a topological pattern of brain atrophy relatively consistent with earlier region-of-interest volumetric studies, indicating a primary limbic and paralimbic distribution of atrophy. The most common structural alteration revealed by VBM is ipsilateral hippocampal and thalamic atrophy (32,58,60-62). The primary area of the thalamus affected appears to be the medial dorsal area (62) (Figure 1), which is a region of the thalamus known to be reciprocally connected with the temporal lobe (63) and anatomically and physiologically altered in animal models of limbic epilepsy (64,65). There is inconsistent evidence on the effect of epilepsy duration on hippocampal grey matter volume in VBM studies (66-69), but more consistent evidence indicating a relationship between increasing thalamic atrophy and epilepsy duration (32,66,67,69). Automated quantitative techniques that permit the analysis of cortical thickness over the whole brain (Figure 2) have revealed bilateral medial and lateral multi-lobar cortical atrophy in patients with unilateral TLE, with some variation in the extent and topological distribution of atrophy (71-79).
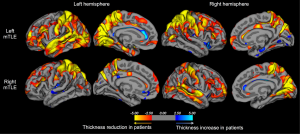
One significant issue with respect to the interpretation of whole-brain voxel based morphometric MRI changes is that there is still insufficient information on the histopathological basis of extrahippocampal, particularly extratemporal lobe structural alterations in TLE. As discussed later, it is important to understand the underlying pathology of MRI changes in TLE so that the biological etiology of postoperative seizures can be resolved. It is known that hippocampal alterations on MRI correlate with histopathologically quantified neuronal loss given that resected hippocampal material can be readily analysed and quantitatively related to preoperative hippocampal volume (27,30,80-83). Similarly, temporal lobe neocortex can be histopathologically examined in patients who undergo anterior temporal lobectomy (ATL). Results have indicated a wider range of lateral temporal lobe neocortical pathology, including gliosis and the presence of heterotopic white matter neurons in some patients with presumed mesial TLE (84). A recent post-mortem study reported significantly reduced neuronal density in the mediodorsal thalamic nucleus in patients with TLE ipsilateral to the side of HS (85), which is consistent with the VBM meta-analysis discussed above (62). However, the inference that MRI-determined grey matter volume loss is a proxy for pathological neuronal loss within cortical regions outside the temporal lobe is currently unsubstantiated.
Given the recent modifications in the classification of epilepsy disorders to consider the importance of brain networks involved in seizure onset, including focal epilepsies (86,87), there has been a new direction of research in TLE to model neuroimaging data in terms of connectivity networks (88). Although this is most eloquently done through analysis of diffusion tensor imaging (DTI) data (see below), inferential analysis of brain structural networks in TLE has been performed using structural MRI covariance, particularly by applying graph theory to segmented and parcellated cortical and subcortical regions-of-interest or correlations in the size, volume or thickness between a single seed region and multiple target regions (79,89-91). For example, Bernhardt et al. evaluated the topographical patterns of cortical atrophy in patients with TLE and observed that, in comparison with controls, patients demonstrated various abnormalities in the network formed by the covariance of regional brain volumes (79). Specifically, patients with epilepsy demonstrated a reorganization of network hubs, as well as changes in global network configuration. Bonilha et al. (92) adopted a similar methodological approach in a recent study examining the organization of cortical covariance networks in children with recently diagnosed epilepsy. This study revealed a similar pattern of topological changes in networks in children with epilepsy, notably due to a significant rearrangement of the regional distribution of network hubs.
One important limitation associated with cortical covariance networks is the fact that only a single network can be determined across a group of subjects. Specifically, one has to assess the covariance across pairs of regions as determined based on the distribution of volumes where each subject is a single data point. Subsequent statistical analyses are then performed based on the putative distribution of data from resampled strategies. Therefore, this method does not permit the assessment of neural architecture at single individual level. Instead, it provides an overview of network abnormalities for TLE as a group.
Diffusion tensor imaging (DTI)
Even though grey matter neurons are presumed to be the generator of seizure activity, white matter is an integral part of the epileptogenic network since axons are the transmission pathways of the brain (93), thus providing the framework for seizure onset and propagation. DTI can provide inferential analysis of neuronal connectivity and structural networks through measurement of water diffusion in the brain, permitting a window into microscopic alterations in patients with epilepsy. Similarly to studies using volumetric MRI, there are many applications of DTI techniques to study brain alterations in refractory TLE. Analysis techniques can be largely categorised into four types: (I) quantification of voxel-based DTI scalar values [e.g., fractional anisotropy (FA) and mean diffusivity (MD)] across the whole brain or within predefined regions-of-interest (e.g., within hippocampus, temporal lobe white matter); (II) probabilistic tractography that generates probabilistic white matter paths between a seed and target region-of-interest; (III) deterministic tractography that permits manual reconstruction of known white matter tracts; and (IV) connectome approaches that typically use tractography analyses to build models of whole brain structural networks and connectivity. A detailed review of DTI studies in refractory TLE is beyond the scope of this article, and the reader is referred to other sources (88,89,93,94). As reviewed by Bernhardt et al. (89), DTI studies consistently show decreased FA in temporo-limbic white matter tracts in groups of patients with TLE, including the fornix, parahippocampal fibers, uncinate fasciculus and cingulum bundles, and in more widespread regions, including the inferior and superior longitudinal fascicles, the internal and external capsules and the corpus callosum. Alterations in tract MD alterations appear more restricted and decrease as a function of anatomical distance to the temporal lobe (89,95). Deep grey matter nuclei also show evidence of diffusion abnormalities, primarily manifested as reduced FA and increased MD of the thalamus (96-99), and an increase in FA of the putamen (96,97).
The opportunity afforded by quantitative DTI to analyse properties of large-scale brain connectivity networks has led to an emerging field in neuroimaging connectomics, which is increasingly being applied to understand the anatomical and physiological basis of TLE (94). Graph theory is applied to quantify and compare properties of networks constructed from nodes (typically multiple cortical and subcortical regions-of-interest obtained from parcellated T1-weighted MRIs) and edges (in the case of DTI for structural connectivity, connecting tracts) distributed across the brain. This is an emergent field within neuroscience and it is particularly promising for epilepsy research, as epilepsy is traditionally considered to be a disease related to abnormal brain networks. Indeed, the neurobiological mechanisms associated with epileptogenesis are tightly linked with aberrant neuronal connectivity.
The concept of epilepsy as a network disease has gained popularity over the past few decades (88,100). In fact, as mentioned above, the notion of epilepsy as a network process has guided the revision of the Classification of Seizures and Epileptic Syndromes (86). This is particularly relevant in the context of TLE since accumulating evidence suggests that extrahippocampal pathology is present in TLE and may configure a network of abnormal structures involved in the generation of seizures (101). Studies employing quantitative imaging methods have consistently demonstrated that patients with TLE exhibit a pattern of structural abnormalities that, albeit invisible on visual inspection of MRI, involve brain structures beyond the hippocampus and the temporal lobe (31-33,58,68,73,102,103). Hence, abnormal extrahippocampal regions can constitute an abnormal network, which may originate and maintain seizures after the removal of the medial temporal lobe and lead to postoperative seizure recurrence (100). Until now, limitations in brain mapping technology have prevented the accurate assessment of individualized patterns of abnormal networks to test the hypothesis suggesting that neural network architecture is associated with surgical responsiveness.
The number of articles demonstrating abnormal connectomes in patients with TLE is still limited given the recent development of the technology. Nonetheless, there is cumulating evidence suggesting that epilepsy, and in particular TLE, is associated with vast connectome abnormalities. For example, Bonilha et al. who pioneered this field, demonstrated that TLE is associated with such abnormalities, notably increased limbic network clustering and efficiency in spite of regional fiber loss (104). DeSalvo et al. subsequently confirmed these findings by demonstrating temporolimbic fiber loss, also paradoxically associated with increased regional network efficiency (105). The neurobiological correlates of these findings are yet to be fully defined, but they likely represent regional changes in connectivity strength, with alterations in the natural balance of associations between regions. By changing the strength with which some regions are associated with each other, the conformation of the limbic network can lead to a relative regional strengthening of connections, even though there is overall fiber loss associated with epilepsy.
MRI and postoperative seizure outcome
Refractory TLE is clearly a systems disorder without a circumscribed brain structural alteration. Understanding how focal and networked structural alterations are related to unsuccessful surgery for refractory temporal lobe seizures is an important research objective and is the focus of this section. This paper is concerned with postoperative seizure outcome, and the use of preoperative imaging to predict postoperative cognitive (106,107), psychiatric (108) and visual (109,110) outcome is beyond the scope of this article. Moreover, this paper is concerned with quantitative MRI and DTI, and the reader is referred to other articles for reviews on other imaging modalities (e.g., functional, metabolic) in refractory focal epilepsy (111,112).
Postoperative MRI: extent of resection
It is naturally important to initially consider whether the amount of potentially epileptogenic tissue resected has a significant influence on postoperative seizure control. Although there are some papers that report such a relationship (113-115), there are others that do not (116-118). It may be that inconsistencies may relate to methodological differences between studies. For example, some studies have (I) compared outcome between patients who underwent ATL and those who underwent selective amygdalohippocampectomy (AH) (119); (II) determined resection length on MRI (114); (III) rated whether a structure was completely, partially or not resected on MRI (115,117,118); or (IV) made assessment of resection parameters during surgery (113,116). Differences in outcome classification, including the classification scale and, more likely, time to postoperative assessment may also explain different findings. A review paper on temporal lobe resection and outcome reported that the extent of resection does not necessarily lead to improved postoperative seizure outcome, that patients with significant hippocampal and amygdaloid remnants may experience excellent postoperative seizure outcomes, and that AH and ATL do not differ in rates of seizure freedom (120). On the contrary, a meta-analysis revealed that ATL was significantly more likely to result in seizure freedom relative to AH (119). However, it must be concluded that class I evidence for the extent and type of resection being related to postoperative seizure outcome is rare (120). What is included in the resection may have a significant influence on outcome; two previous studies reported that the extent of entorhinal and parahippocampal resection is significantly related to outcome (121,122). Such findings indicate that preoperative identification of entorhinal and parahippocampal abnormalities may potentially identify a particular subtype of refractory TLE that is less responsive to circumscribed resections (101).
Preoperative volumetric MRI
Given that the hippocampus is considered the primary seizure generator in TLE (123)—or a primary node in an epileptiform network—it is logical to start by rigorously examining the morphology and pathology of the hippocampus using quantitative MRI. Many preoperative evaluation programs include quantitative analysis of hippocampal volume, and there have been some studies that have analyzed such data with respect to postoperative seizure outcome. Using these approaches, Jack et al. (124) reported that a larger hippocampal volume ipsilateral to resection was related to a poorer outcome in a mixed cohort of patients with TLE with and without HS, and Jutila et al. (125) reported that hippocampal volume reduction of at least one standard deviation from the mean of controls was related to improved seizure outcome. Using a classification of HA or no HA based on volumetry, patients achieving an excellent postoperative seizure outcome were significantly more likely to have HA based on a simple classification analysis (126-128). Patients with bilateral or no HA based on volumetry are significantly less likely to attain seizure freedom after surgery relative to patients with clear unilateral HA (10), although satisfactory postoperative outcomes in patients with bilaterally symmetric hippocampal volumes are achievable (129). Conversely, other studies have found no relationship with global hippocampal volume and postoperative seizure control when outcome groups were retrospectively compared (130-132). Although it is generally accepted that the presence of HS leads to improved postoperative seizure control relative to patients with unremarkable MRI, up to 40% of TLE patients with neuroradiological diagnosed unilateral HS—manifested as HA for the majority of patients—will continue to experience postoperative seizures (2). Therefore hippocampal volumetry is unlikely to reliably prospectively stratify individual patients with TLE and HS into probable outcome groups.
Soon after the introduction of increasingly sophisticated quantitative MRI techniques there was the application of automated approaches to relate preoperative hippocampal and extrahippocampal alterations to postoperative outcome in TLE. Studies have reported regional surface (133) and density (134) alterations of the hippocampus contralateral to intended resection in patients with persistent postoperative seizures relative to those rendered seizure free. A previous study reported that 25% of patients with TLE and HS who continued to experience seizures had postoperative epileptiform contributions from the contralateral temporal lobe (135). Bitemporal epileptogenic activity previously undetected during presurgical evaluation could therefore contribute to persistent postoperative seizures in some patients.
In a small group of patients with left TLE and pathologically proven HS, Keller et al. (134) reported that patients with persistent postoperative seizures had significantly reduced grey matter density of the posterior hippocampal region relative to those rendered seizure free using VBM. The authors suggested that given that temporal lobe resections typically leave a posterior hippocampal remnant, it may be that the hippocampal remnant is epileptogenic, remains functionally connected after surgery, and contributes to persistent postoperative seizures. This finding has been recently replicated using a hippocampal surface mapping technique in a larger cohort of patients with TLE and HS, and also confirmed that global hippocampal volume on MRI was not a predictor of postoperative seizure outcome (70). Patients with a posterior mesial temporal lobe seizure onset are more likely to experience persistent postoperative seizures relative to patients with an anterior mesial temporal lobe onset (136,137), and patients with a poor outcome are more likely to have a distribution of neuronal cell loss throughout the anterior-posterior extent of the hippocampus relative to patients with an excellent outcome who had cell loss confined to anterior—and thus resected—regions of the hippocampus (138). However, the finding of posterior HA being preferentially observed in patients with persistent seizures has not been replicated in a study that quantified the volume of the hippocampal head, body and tail in patients with TLE (132), and a previous electrophysiological study indicated that the hippocampal remnant after surgery was the cause of postoperative seizures in only 5% of patients with TLE and HS (135).
Within group comparison morphometric studies, there are reports of increasing extrahippocampal structural alterations in patients with persistent postoperative seizures relative to those rendered seizure free (70,71,90,134,139-141). Extrahippocampal involvement in postoperative seizures has been related to increasing atrophy of the contralateral entorhinal cortex (141), atrophy of the temporopolar and insular cortices (71), atrophy of the parahippocampal region bilaterally (134), alterations in thalamotemporal structure (70), increased whole-brain structural network disruption (90), increased number of anatomically non-specific extrahippocampal abnormalities (139), and generalized grey and white matter atrophy (140), on preoperative MRI. Inferences made from most of these studies are that patients who have an excellent seizure outcome have cerebral alterations mostly restricted to the mesial temporal lobe being resected or disconnected. This suggestion, however, would need to be reconciled with reports of bilateral extrahippocampal structural alterations in patients with well-characterised unilateral TLE and ipsilateral HS who are likely to have standard (up to 60-70% seizure freedom) postoperative seizure outcome rates (97). This has led some to suggest that bilateral temporal and extratemporal structural alterations in patients with TLE and neuroradiological evidence of unilateral HS is common and may not be predictive of poor surgical outcome (142).
It is likely that different findings on the relationship between preoperative brain structure on MRI and postoperative seizure outcome are due to a number of methodological factors. Table 2 presents a breakdown of methodological information across selected studies that have addressed the preoperative neuroanatomical correlates of postoperative seizures using quantitative MRI techniques. From this table it emerges that no single methodological parameter is consistent across studies. In particular, studies differ substantially in terms of (I) the characterization of patients; (II) number of participants studied; (III) surgical approach; (IV) classification of seizure freedom; (V) MRI acquisition; (VI) the area of the brain assessed; and (VII) the morphometric technique employed. In samples of patients with TLE who have not been preselected according to hippocampal pathology (i.e., to include those with no MRI changes, or those with lesional pathology other than HS), HA on MRI ipsilateral to the side of seizure onset is consistently associated with an improved postoperative seizure outcome. However, this is not the case when patients are enrolled into studies on the basis of electrophysiological evidence of TLE and HS, which are the strongest indicators for mesial TLE (mTLE). In these cases, it may be that extrahippocampal or bitemporal alterations may be associated with a poor outcome, or perhaps that a so-far unidentified subtype of mTLE that is less amenable to resective surgery exists.
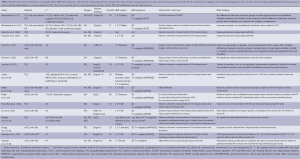
Full table
DTI: connectivity and networks
There is abundant evidence to suggest that voxel-based scalars of water diffusion, as well as DTI fiber tractography, are abnormal in patients with TLE, notably within areas that are functionally or anatomically connected with the medial temporal regions. Nonetheless, the relevance of these findings towards clinical outcomes is not yet fully defined. A growing body of evidence suggests that extrahippocampal abnormalities are indeed a crucial determinant of neuropsychological performance. Winston et al. demonstrated that white matter integrity in a frontoparietal network and in the contralateral temporal lobe is associated with working memory performance (144). McDonald et al. reported that structural compromise to multiple DTI streamlines tracts is associated with memory and language impairments in patients with TLE (145) and memory performance is better predicted when DTI data regarding white matter integrity is combined with gray matter information from conventional morphometric measures (146). Interestingly, the one to one relationship between memory type deficits and anatomical pathways has not been fully determined. Certainly, medial temporal outflow pathways such as the fornix may play an important role (147), but the overall distribution across extratemporal regions is likely more widespread, or perhaps variable across individuals.
The relationship between seizure control and white matter integrity demonstrated by DTI is starting to be explored in a systematic way. Gonçalves Pereira demonstrated that hippocampal MD asymmetry indices are higher in patients with optimal surgical outcome (148). The relationship between extrahippocampal abnormalities and outcome is also likely important, as structural connectivity demonstrates a reorganization of strong temporal-extratemporal connections in patients with suboptimal outcome (149). While these are preliminary findings, there appears to be a growing body of evidence to suggest that white matter organization supports the clinical profile of epilepsy and seizure control.
Future work
The development of imaging prognostic classifiers that can stratify individual patients according to likely outcome is an important future direction. It may be that quantitative MRI alone cannot achieve this, particularly using the current resolution of clinical MRI systems, and that multimodal imaging (combining structure, function and metabolism) will be more informative. There is evidence indicating that temporal lobe (150,151) and extratemporal lobe (152,153) fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) hypometabolism is an indicator for postoperative seizure outcome. Magnetic resonance spectroscopy (MRS) has been shown to have excellent lateralising value in patients with TLE, particularly in conjunction with hippocampal volumetry (154), shows promise in predicting pharmacoresistance (155), but has mixed evidence for the ability to predict postoperative outcome in unilateral TLE (128,156-158). Resting-state functional MRI may also offer promise for in the prediction of postoperative outcome (159). Whether these multimodal quantitative imaging techniques can be used alongside quantitative MRI and DTI methods for individual prognostic classification remains to be seen.
Classical HS is characterized by preferential neuron loss and gliosis in CA1, and also to a lesser extent of CA4 and CA3, with relative resistance of CA2 neurons (160,161). However, over half of patients with well-characterized refractory mesial TLE have neuronal loss throughout all CA sectors (162). It has been demonstrated that patients with classical and total HS, who make up the vast majority of patients with TLE and HS, have a greater chance of seizure freedom after surgery relative to patients with no histopathological evidence of HS, or atypical patterns of HS manifested as circumscribed loss of neurons in CA1 or CA4 (162,163). Patients with atypical patterns of HS may be considered to have a particular subtype of TLE that is resistant to conventional resective surgery. An important future quantitative MRI research endeavor would therefore be an attempt to identify atypical patterns of HS on preoperative MRI as this may suggest a potential poor postoperative prognosis in those patients. To our knowledge, there have been no quantitative MRI studies attempting to correlate atypical patterns of HS prior to surgery with postoperative outcome, despite some progress in the identification of hippocampal subfields using high-field MRI (164) and probabilistic mapping (165).
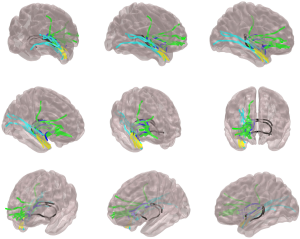
Regarding white matter networks and TLE, there are undoubtedly several questions that remain to be answered, including whether (I) abnormal networks are the same for all patients with TLE; (II) there are crucial areas within the network where abnormalities lead to surgical refractoriness; (III) pharmacological intractability is also a function of abnormal networks and can be tracked over the time to provide a measure of long-term trajectory and guide management decisions; (IV) abnormalities are reversible after successful treatment; and (V) white matter topography can be used as an individualized measurement of clinical course. It is conceivable that several of these questions may be adequately answered by DTI analyses. An example of possible anatomical pathways of seizure spread is demonstrated in Figure 3. By examining the structural properties of these pathways, alone or in combination, structural connectivity may provide information regarding the architectural variability across individuals with epilepsy. Importantly, this information may be used to summarize the degree and extent of network abnormalities and possibly be used to provide individualized measures of operative outcome.
Conclusions
Refractory TLE is characterized by networked brain structural alterations that exist primarily in limbic and paralimbic regions, which have been reproducibly demonstrated across studies using quantitative MRI and DTI techniques. Some of these structural and connectivity alterations may have significance for why some patients respond well to resective surgery and others do not. An important future goal is to use quantitative imaging techniques to identify a particular subtype of TLE that is particularly resistant to conventional temporal lobe surgery. Whether this information can be applied to stratify individual patients according to predicted outcome remains to be determined.
Acknowledgements
Funding: SSK is supported by a UK Medical Research Council grant (Grant Number MR/K023152/1).
Disclosure: The authors declare no conflict of interest.
References
- Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol 2012;8:669-77. [PubMed]
- Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, Ebner A. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 2005;128:395-404. [PubMed]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-8. [PubMed]
- Engel J Jr, Van Ness P, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J Jr. eds. Surgical treatment of the epilepsies. New York: Reven Press, 1993:609-21.
- Hennessy MJ, Elwes RD, Honavar M, Rabe-Hesketh S, Binnie CD, Polkey CE. Predictors of outcome and pathological considerations in the surgical treatment of intractable epilepsy associated with temporal lobe lesions. J Neurol Neurosurg Psychiatry 2001;70:450-8. [PubMed]
- Polkey CE. Clinical outcome of epilepsy surgery. Curr Opin Neurol 2004;17:173-8. [PubMed]
- Dam M. Epilepsy surgery. Acta Neurol Scand 1996;94:81-7. [PubMed]
- McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004;127:2018-30. [PubMed]
- de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 2011;378:1388-95. [PubMed]
- Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, Poulin N, Arnold DL, Olivier A. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol 1996;40:446-50. [PubMed]
- Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, Bladin PF, Hopper JL. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology 1995;45:1358-63. [PubMed]
- McIntosh AM, Wilson SJ, Berkovic SF. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia 2001;42:1288-307. [PubMed]
- Radhakrishnan K, So EL, Silbert PL, Jack CR Jr, Cascino GD, Sharbrough FW, O'Brien PC. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology 1998;51:465-71. [PubMed]
- Bengzon AR, Rasmussen T, Gloor P, Dussault J, Stephens M. Prognostic factors in the surgical treatment of temporal lobe epileptics. Neurology 1968;18:717-31. [PubMed]
- Schulz R, Lüders HO, Hoppe M, Tuxhorn I, May T, Ebner A. Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia 2000;41:564-70. [PubMed]
- Specht U, May T, Schulz R, Rohde M, Ebner A, Schmidt RC, Schütz M, Wolf P. Cerebellar atrophy and prognosis after temporal lobe resection. J Neurol Neurosurg Psychiatry 1997;62:501-6. [PubMed]
- Abou-Khalil B, Andermann E, Andermann F, Olivier A, Quesney LF. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia 1993;34:878-83. [PubMed]
- Janszky J, Schulz R, Ebner A. Clinical features and surgical outcome of medial temporal lobe epilepsy with a history of complex febrile convulsions. Epilepsy Res 2003;55:1-8. [PubMed]
- Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology 2008;71:413-8. [PubMed]
- Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 2005;46:1273-9. [PubMed]
- Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, Langfitt JT, Walczak TS, Pacia SV; Multicenter Study of Epilepsy Surgery. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology 2005;65:912-8. [PubMed]
- Kilpatrick C, Cook M, Matkovic Z, O'Brien T, Kaye A, Murphy M. Seizure frequency and duration of epilepsy are not risk factors for postoperative seizure outcome in patients with hippocampal sclerosis. Epilepsia 1999;40:899-903. [PubMed]
- Winston GP, Cardoso MJ, Williams EJ, Burdett JL, Bartlett PA, Espak M, Behr C, Duncan JS, Ourselin S. Automated hippocampal segmentation in patients with epilepsy: available free online. Epilepsia 2013;54:2166-73. [PubMed]
- Mackay CE, Webb JA, Eldridge PR, Chadwick DW, Whitehouse GH, Roberts N. Quantitative magnetic resonance imaging in consecutive patients evaluated for surgical treatment of temporal lobe epilepsy. Magn Reson Imaging 2000;18:1187-99. [PubMed]
- Jack CR Jr. MRI-based hippocampal volume measurements in epilepsy. Epilepsia 1994;35 Suppl 6:S21-9. [PubMed]
- Cascino GD. Clinical correlations with hippocampal atrophy. Magn Reson Imaging 1995;13:1133-6. [PubMed]
- Cascino GD, Jack CR Jr, Parisi JE, Sharbrough FW, Hirschorn KA, Meyer FB, Marsh WR, O'Brien PC. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 1991;30:31-6. [PubMed]
- Jack CR Jr, Sharbrough FW, Twomey CK, Cascino GD, Hirschorn KA, Marsh WR, Zinsmeister AR, Scheithauer B. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 1990;175:423-9. [PubMed]
- Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990;40:1869-75. [PubMed]
- Watson C, Jack CR Jr, Cendes F. Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol 1997;54:1521-31. [PubMed]
- Bonilha L, Kobayashi E, Rorden C, Cendes F, Li LM. Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2003;74:1627-30. [PubMed]
- Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage 2005;25:1016-21. [PubMed]
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 2003;126:462-9. [PubMed]
- Bernasconi N, Bernasconi A, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Morphometric MRI analysis of the parahippocampal region in temporal lobe epilepsy. Ann N Y Acad Sci 2000;911:495-500. [PubMed]
- Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology 1999;52:1870-6. [PubMed]
- Bernasconi N, Bernasconi A, Caramanos Z, Dubeau F, Richardson J, Andermann F, Arnold DL. Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology 2001;56:1335-9. [PubMed]
- Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain 2001;124:167-75. [PubMed]
- Dreifuss S, Vingerhoets FJ, Lazeyras F, Andino SG, Spinelli L, Delavelle J, Seeck M. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology 2001;57:1636-41. [PubMed]
- Pulsipher DT, Seidenberg M, Morton JJ, Geary E, Parrish J, Hermann B. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav 2007;11:442-9. [PubMed]
- DeCarli C, Hatta J, Fazilat S, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998;43:41-5. [PubMed]
- Theodore WH, Gaillard WD. Neuroimaging and the progression of epilepsy. Prog Brain Res 2002;135:305-13. [PubMed]
- Fuerst D, Shah J, Kupsky WJ, Johnson R, Shah A, Hayman-Abello B, Ergh T, Poore Q, Canady A, Watson C. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology 2001;57:184-8. [PubMed]
- Salmenperä T, Kälviäinen R, Partanen K, Pitkänen A. Hippocampal damage caused by seizures in temporal lobe epilepsy. Lancet 1998;351:35. [PubMed]
- Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol 1999;45:568-76. [PubMed]
- Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 1999;52:132-6. [PubMed]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology 2005;65:223-8. [PubMed]
- Kälviäinen R, Salmenperä T, Partanen K, Vainio P, Riekkinen P, Pitkänen A. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology 1998;50:1377-82. [PubMed]
- Kälviäinen R, Salmenperä T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog Brain Res 2002;135:279-95. [PubMed]
- Cendes F, Andermann F, Gloor P, Lopes-Cendes I, Andermann E, Melanson D, Jones-Gotman M, Robitaille Y, Evans A, Peters T. Atrophy of mesial structures in patients with temporal lobe epilepsy: cause or consequence of repeated seizures? Ann Neurol 1993;34:795-801. [PubMed]
- Davies KG, Hermann BP, Dohan FC Jr, Foley KT, Bush AJ, Wyler AR. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res 1996;24:119-26. [PubMed]
- Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GD. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol 2002;51:641-4. [PubMed]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol 2003;53:413-6. [PubMed]
- Liu RS, Lemieux L, Bell GS, Bartlett PA, Sander JW, Sisodiya SM, Shorvon SD, Duncan JS. A longitudinal quantitative MRI study of community-based patients with chronic epilepsy and newly diagnosed seizures: methodology and preliminary findings. Neuroimage 2001;14:231-43. [PubMed]
- Liu RS, Lemieux L, Bell GS, Hammers A, Sisodiya SM, Bartlett PA, Shorvon SD, Sander JW, Duncan JS. Progressive neocortical damage in epilepsy. Ann Neurol 2003;53:312-24. [PubMed]
- Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol 2003;458:272-92. [PubMed]
- Cendes F. Progressive hippocampal and extrahippocampal atrophy in drug resistant epilepsy. Curr Opin Neurol 2005;18:173-7. [PubMed]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage 2000;11:805-21. [PubMed]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 2008;49:741-57. [PubMed]
- Kakeda S, Korogi Y. The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and Alzheimer's disease/mild cognitive impairment: a review. Neuroradiology 2010;52:711-21. [PubMed]
- Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy. Expert Rev Neurother 2010;10:975-84. [PubMed]
- Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res 2012;98:97-103. [PubMed]
- Barron DS, Fox PM, Laird AR, Robinson JL, Fox PT. Thalamic medial dorsal nucleus atrophy in medial temporal lobe epilepsy: A VBM meta-analysis. Neuroimage Clin 2012;2:25-32. [PubMed]
- Keller SS, O'Muircheartaigh J, Traynor C, Towgood K, Barker GJ, Richardson MP. Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia 2014;55:306-15. [PubMed]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia 2001;42:967-78. [PubMed]
- Bertram EH, Scott C. The pathological substrate of limbic epilepsy: neuronal loss in the medial dorsal thalamic nucleus as the consistent change. Epilepsia 2000;41 Suppl 6:S3-8. [PubMed]
- Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 2002;73:648-55. [PubMed]
- Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009;73:834-42. [PubMed]
- Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage 2004;23:717-23. [PubMed]
- Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage 2006;32:1070-9. [PubMed]
- Keller SS, Richardson MP, O’Muircheartaigh J, Schoene-Bake JC, Elger C, Weber B. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Human Brain Mapping 2015; [PubMed]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776-84. [PubMed]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J Jr, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex 2007;17:2007-18. [PubMed]
- McDonald CR, Hagler DJ Jr, Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, Dale AM, Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 2008;49:794-803. [PubMed]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009;72:1747-54. [PubMed]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage 2009;46:353-9. [PubMed]
- Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15:445-51. [PubMed]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, Cardenas-Nicolson V, Weiner MW. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia 2010;51:1436-45. [PubMed]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, Iragui VJ, Hagler DJ Jr, Halgren E, McDonald CR. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011;52:2257-66. [PubMed]
- Bernhardt BC, Bernasconi N, Kim H, Bernasconi A. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012;78:129-36. [PubMed]
- Bronen RA, Cheung G, Charles JT, Kim JH, Spencer DD, Spencer SS, Sze G, McCarthy G. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am J Neuroradiol 1991;12:933-40. [PubMed]
- Lencz T, McCarthy G, Bronen RA, Scott TM, Inserni JA, Sass KJ, Novelly RA, Kim JH, Spencer DD. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 1992;31:629-37. [PubMed]
- Lee N, Tien RD, Lewis DV, Friedman AH, Felsberg GJ, Crain B, Hulette C, Osumi AK, Smith JS, VanLandingham KE, et al. Fast spin-echo, magnetic resonance imaging-measured hippocampal volume: correlation with neuronal density in anterior temporal lobectomy patients. Epilepsia 1995;36:899-904. [PubMed]
- Watson C, Cendes F, Fuerst D, Dubeau F, Williamson B, Evans A, Andermann F. Specificity of volumetric magnetic resonance imaging in detecting hippocampal sclerosis. Arch Neurol 1997;54:67-73. [PubMed]
- Nishio S, Morioka T, Hisada K, Fukui M. Temporal lobe epilepsy: a clinicopathological study with special reference to temporal neocortical changes. Neurosurg Rev 2000;23:84-9. [PubMed]
- Sinjab B, Martinian L, Sisodiya SM, Thom M. Regional thalamic neuropathology in patients with hippocampal sclerosis and epilepsy: a postmortem study. Epilepsia 2013;54:2125-33. [PubMed]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51:676-85. [PubMed]
- Berg AT, Scheffer IE. New concepts in classification of the epilepsies: entering the 21st century. Epilepsia 2011;52:1058-62. [PubMed]
- Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 2012;83:1238-48. [PubMed]
- Bernhardt BC, Hong S, Bernasconi A, Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 2013;7:624. [PubMed]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 2011;21:2147-57. [PubMed]
- Bernhardt BC, Worsley KJ, Besson P, Concha L, Lerch JP, Evans AC, Bernasconi N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage 2008;42:515-24. [PubMed]
- Bonilha L, Tabesh A, Dabbs K, Hsu DA, Stafstrom CE, Hermann BP, Lin JJ. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp 2014;35:3661-72. [PubMed]
- Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 2011;52 Suppl 4:32-34. [PubMed]
- Haneef Z, Chiang S. Clinical correlates of graph theory findings in temporal lobe epilepsy. Seizure 2014;23:809-18. [PubMed]
- Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012;79:455-62. [PubMed]
- Keller SS, Ahrens T, Mohammadi S, Gerdes JS, Möddel G, Kellinghaus C, Kugel H, Weber B, Ringelstein EB, Deppe M. Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. J Neuroimaging 2013;23:352-9. [PubMed]
- Keller SS, Schoene-Bake JC, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One 2012;7:e46791. [PubMed]
- Gong G, Concha L, Beaulieu C, Gross DW. Thalamic diffusion and volumetry in temporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Res 2008;80:184-93. [PubMed]
- Kim CH, Koo BB, Chung CK, Lee JM, Kim JS, Lee SK. Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: a diffusion tensor imaging study. Epilepsy Res 2010;90:21-7. [PubMed]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43:219-27. [PubMed]
- Bonilha L, Martz GU, Glazier SS, Edwards JC. Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 2012;53:1-6. [PubMed]
- Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 2004;61:1379-84. [PubMed]
- Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 2002;16:23-31. [PubMed]
- Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 2012;83:903-9. [PubMed]
- DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered structural connectome in temporal lobe epilepsy. Radiology 2014;270:842-8. [PubMed]
- Trenerry MR, Jack CR Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 1993;43:1800-5. [PubMed]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Barker GJ, Thompson PJ, Koepp MJ, Duncan JS. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry 2008;79:327-30. [PubMed]
- Wrench JM, Wilson SJ, Bladin PF, Reutens DC. Hippocampal volume and depression: insights from epilepsy surgery. J Neurol Neurosurg Psychiatry 2009;80:539-44. [PubMed]
- Borius PY, Roux FE, Valton L, Sol JC, Lotterie JA, Berry I. Can DTI fiber tracking of the optic radiations predict visual deficit after surgery? Clin Neurol Neurosurg 2014;122:87-91. [PubMed]
- Winston GP, Daga P, Stretton J, Modat M, Symms MR, McEvoy AW, Ourselin S, Duncan JS. Optic radiation tractography and vision in anterior temporal lobe resection. Ann Neurol 2012;71:334-41. [PubMed]
- Koepp MJ, Woermann FG. Imaging structure and function in refractory focal epilepsy. Lancet Neurol 2005;4:42-53. [PubMed]
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat Rev Neurol 2010;6:537-50. [PubMed]
- Salanova V, Andermann F, Rasmussen T, Olivier A, Quesney L. The running down phenomenon in temporal lobe epilepsy. Brain 1996;119:989-96. [PubMed]
- Joo EY, Han HJ, Lee EK, Choi S, Jin JH, Kim JH, Tae WS, Seo DW, Hong SC, Lee M, Hong SB. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure 2005;14:541-51. [PubMed]
- Bonilha L, Kobayashi E, Mattos JP, Honorato DC, Li LM, Cendes F. Value of extent of hippocampal resection in the surgical treatment of temporal lobe epilepsy. Arq Neuropsiquiatr 2004;62:15-20. [PubMed]
- Hardy SG, Miller JW, Holmes MD, Born DE, Ojemann GA, Dodrill CB, Hallam DK. Factors predicting outcome of surgery for intractable epilepsy with pathologically verified mesial temporal sclerosis. Epilepsia 2003;44:565-8. [PubMed]
- Kanner AM, Kaydanova Y, deToledo-Morrell L, Morrell F, Smith MC, Bergen D, Pierre-Louis SJ, Ristanovic R. Tailored anterior temporal lobectomy. Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol 1995;52:173-8. [PubMed]
- Jack CR Jr, Sharbrough FW, Marsh WR. Use of MR imaging for quantitative evaluation of resection for temporal lobe epilepsy. Radiology 1988;169:463-8. [PubMed]
- Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, Wiebe S. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology 2013;80:1669-76. [PubMed]
- Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia 2008;49:1296-307. [PubMed]
- Bonilha L, Yasuda CL, Rorden C, Li LM, Tedeschi H, de Oliveira E, Cendes F. Does resection of the medial temporal lobe improve the outcome of temporal lobe epilepsy surgery? Epilepsia 2007;48:571-8. [PubMed]
- Siegel AM, Wieser HG, Wichmann W, Yasargil GM. Relationships between MR-imaged total amount of tissue removed, resection scores of specific mediobasal limbic subcompartments and clinical outcome following selective amygdalohippocampectomy. Epilepsy Res 1990;6:56-65. [PubMed]
- Avoli M. The epileptic hippocampus revisited: back to the future. Epilepsy Curr 2007;7:116-8. [PubMed]
- Jack CR Jr, Sharbrough FW, Cascino GD, Hirschorn KA, O'Brien PC, Marsh WR. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 1992;31:138-46. [PubMed]
- Jutila L, Immonen A, Mervaala E, Partanen J, Partanen K, Puranen M, Kälviäinen R, Alafuzoff I, Hurskainen H, Vapalahti M, Ylinen A. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry 2002;73:486-94. [PubMed]
- Cascino GD, Trenerry MR, Sharbrough FW, So EL, Marsh WR, Strelow DC. Depth electrode studies in temporal lobe epilepsy: relation to quantitative magnetic resonance imaging and operative outcome. Epilepsia 1995;36:230-5. [PubMed]
- Cascino GD, Trenerry MR, Jack CR Jr, Dodick D, Sharbrough FW, So EL, Lagerlund TD, Shin C, Marsh WR. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia 1995;36:692-6. [PubMed]
- Knowlton RC, Laxer KD, Ende G, Hawkins RA, Wong ST, Matson GB, Rowley HA, Fein G, Weiner MW. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol 1997;42:829-37. [PubMed]
- Jack CR Jr, Trenerry MR, Cascino GD, Sharbrough FW, So EL, O'Brien PC. Bilaterally symmetric hippocampi and surgical outcome. Neurology 1995;45:1353-8. [PubMed]
- Quigg M, Bertram EH, Jackson T, Laws E. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia 1997;38:588-94. [PubMed]
- Mueller CA, Scorzin J, von Lehe M, Fimmers R, Helmstaedter C, Zentner J, Lehmann TN, Meencke HJ, Schulze-Bonhage A, Schramm J. Seizure outcome 1 year after temporal lobe epilepsy: an analysis of MR volumetric and clinical parameters. Acta Neurochir (Wien) 2012;154:1327-36. [PubMed]
- Goh YY, Schoene-Bake JC, Marson AG, Richardson MP, Weber B, Keller SS. Hippocampal volume and postsurgical outcome in intractable temporal lobe epilepsy. Epilepsy Currents 2014;14:232-3.
- Lin JJ, Salamon N, Dutton RA, Lee AD, Geaga JA, Hayashi KM, Toga AW, Engel J Jr, Thompson PM. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology 2005;65:1094-7. [PubMed]
- Keller SS, Cresswell P, Denby C, Wieshmann U, Eldridge P, Baker G, Roberts N. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res 2007;74:131-9. [PubMed]
- Hennessy MJ, Elwes RD, Binnie CD, Polkey CE. Failed surgery for epilepsy. A study of persistence and recurrence of seizures following temporal resection. Brain 2000;123:2445-66. [PubMed]
- Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure 2000;9:407-11. [PubMed]
- Prasad A, Pacia SV, Vazquez B, Doyle WK, Devinsky O. Extent of ictal origin in mesial temporal sclerosis patients monitored with subdural intracranial electrodes predicts outcome. J Clin Neurophysiol 2003;20:243-8. [PubMed]
- Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia 1984;25:729-40. [PubMed]
- Sisodiya SM, Moran N, Free SL, Kitchen ND, Stevens JM, Harkness WF, Fish DR, Shorvon SD. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Ann Neurol 1997;41:490-6. [PubMed]
- Yasuda CL, Valise C, Saúde AV, Pereira AR, Pereira FR, Ferreira Costa AL, Morita ME, Betting LE, Castellano G, Mantovani Guerreiro CA, Tedeschi H, de Oliveira E, Cendes F. Dynamic changes in white and gray matter volume are associated with outcome of surgical treatment in temporal lobe epilepsy. Neuroimage 2010;49:71-9. [PubMed]
- Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology 2013;81:1840-7. [PubMed]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 2006;47:1360-3. [PubMed]
- Feis DL, Schoene-Bake JC, Elger C, Wagner J, Tittgemeyer M, Weber B. Prediction of post-surgical seizure outcome in left mesial temporal lobe epilepsy. Neuroimage Clin 2013;2:903-11. [PubMed]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Thompson PJ, Duncan JS. Structural correlates of impaired working memory in hippocampal sclerosis. Epilepsia 2013;54:1143-53. [PubMed]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 2008;71:1869-76. [PubMed]
- McDonald CR, Leyden KM, Hagler DJ, Kucukboyaci NE, Kemmotsu N, Tecoma ES, Iragui VJ. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex 2014;58:139-50. [PubMed]
- Alexander RP, Concha L, Snyder TJ, Beaulieu C, Gross DW. Correlations between Limbic White Matter and Cognitive Function in Temporal-Lobe Epilepsy, Preliminary Findings. Front Aging Neurosci 2014;6:142. [PubMed]
- Gonçalves Pereira PM, Oliveira E, Rosado P. Apparent diffusion coefficient mapping of the hippocampus and the amygdala in pharmaco-resistant temporal lobe epilepsy. AJNR Am J Neuroradiol 2006;27:671-83. [PubMed]
- Bonilha L, Helpern JA, Sainju R, Nesland T, Edwards JC, Glazier SS, Tabesh A. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 2013;81:1704-10. [PubMed]
- Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, O'Brien TJ. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain 2007;130:548-60. [PubMed]
- Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, Beam C, Coleman RE, Friedman AH. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology 1993;43:1088-92. [PubMed]
- Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, Kim SE. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging 2003;30:581-7. [PubMed]
- Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, Somerville E, Mohamed A. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia 2010;51:1365-73. [PubMed]
- Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 1997;42:737-46. [PubMed]
- Campos BA, Yasuda CL, Castellano G, Bilevicius E, Li LM, Cendes F. Proton MRS may predict AED response in patients with TLE. Epilepsia 2010;51:783-8. [PubMed]
- Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy. Evidence from proton MR spectroscopic imaging. Neurology 1997;49:1525-33. [PubMed]
- Kuzniecky R, Hugg J, Hetherington H, Martin R, Faught E, Morawetz R, Gilliam F. Predictive value of 1H MRSI for outcome in temporal lobectomy. Neurology 1999;53:694-8. [PubMed]
- Antel SB, Li LM, Cendes F, Collins DL, Kearney RE, Shinghal R, Arnold DL. Predicting surgical outcome in temporal lobe epilepsy patients using MRI and MRSI. Neurology 2002;58:1505-12. [PubMed]
- Morgan VL, Sonmezturk HH, Gore JC, Abou-Khalil B. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia 2012;53:1628-35. [PubMed]
- Bratz E. Ammonshornbefunde bei Epileptikern. Arch Psychiatr Nervenkr 1899;32:820-35.
- de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia 2003;44:677-87. [PubMed]
- Blümcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstöck J, Stefan H, Hildebrandt M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 2007;113:235-44. [PubMed]
- Thom M, Liagkouras I, Elliot KJ, Martinian L, Harkness W, McEvoy A, Caboclo LO, Sisodiya SM. Reliability of patterns of hippocampal sclerosis as predictors of postsurgical outcome. Epilepsia 2010;51:1801-8. [PubMed]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4 Tesla: preliminary results. Epilepsia 2009;50:1474-83. [PubMed]
- Schoene-Bake JC, Keller SS, Niehusmann P, Volmering E, Elger C, Deppe M, Weber B. In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Hum Brain Mapp 2014;35:4718-28. [PubMed]


