Daily patient setup error in prostate image guided radiation therapy with fiducial-based kilovoltage onboard imaging and conebeam computed tomography
Introduction
Since the field’s inception over a century ago, radiation oncology has been faced with the fundamental challenge of delivering radiotherapy to malignant tissue while sparing surrounding healthy organs. In order to address this issue, researchers have delved into image-guided radiation therapy (IGRT), which refers to the integration of radiation planning, patient positioning, and treatment delivery with new and emerging image-based tumor definition methods (1). While advances in tumor volume delineation and the practice of IGRT have led to more precise treatments, the need for increased accuracy in beam targeting remains (2). Greater accuracy would allow conforming of the planning target volume (PTV) more closely to the clinical target volume (CTV), which would reduce the volume of healthy tissue irradiated. In turn, this could allow radiation dose escalation resulting in better local control with decreased associated morbidity. The benefit of dose escalation with conformal radiation delivery has already been well-demonstrated in the treatment of prostate cancer (3).
One of the commonly practiced method for verifying interfraction changes involves the comparison of megavoltage (MV) X-ray portal images to the reference kilovoltage (kV) X-ray images obtained during computed tomography (CT) simulations or to digitally reconstructed radiographs (DRRs) (4). To circumvent the poorer resolution of MV imaging, a number of studies have been conducted implementing planar and volumetric kV imaging (5,6). The On-Board Imager™ (OBI) has been commonly used to provide this type of imaging. It consists of a diagnostic X-ray tube and kV flat-panel imager mounted on the treatment machine gantry via robotic arms that operate along three axes of motion. In recent years, the OBI has been used to verify patient positioning via two dimensions—planar orthogonal kV radiographs, and via three dimensions—kV conebeam CT (CBCT).
kV CBCT allows for the reconstruction of a three-dimensional (3D) CT image from data collected during a single gantry rotation around a stationary patient (7). Such an imaging system has been shown to be capable of producing soft tissue images with excellent spatial resolution in a seamless, efficient manner (8). Thus, it has often been used preferentially over paired orthogonal kV images in cases where 3D soft tissue detail is important for patient position verification (5). It has also been used when treating small targets without fiducial markers, when using a small number of treatment fractions, and when performing adaptive radiotherapy (5). Still, while kV CBCT provides better soft tissue contrast, its use could be limited by the increase in radiation session length it requires as well as the greater dose it delivers to the patient in comparison to planar imaging (9).
In cases of prostate cancer, both of these image registration methods have provided daily image guidance in an attempt to more accurately deliver radiation (4,6). The location and mobility of the prostate makes its accurate localization using external markers such as skin tattoos and bony anatomy difficult. While ultrasound has also been widely utilized for localization of the prostate, it was found to be less accurate than kV imaging with fiducial markers (10). Fiducials—radioopaque typically gold, cylindrical seeds—are implanted into the prostate via needle under transrectal ultrasound guidance (11). Imaging techniques using fiducials located within the prostate allows for more accurate targeting of the mobile organ and reduces interfractional variation of radiation. Greer et al. reported offline bony anatomy systematic errors of 1.6, 2.5 and 4.4 mm in the right-left (RL), superior-inferior (SI), and anterior-posterior (AP) directions, respectively (12). McNair et al. reported offline bony anatomy systemic errors of 1.3, 1.9, and 2.5 mm and online fiducial systemic errors of 0.9, 1.1, and 1.0 mm in the RL, SI, and AP directions, respectively (13). Both studies also calculated the margins to account for set-up error for each technique which was of 6, 9, and 13 mm (12) and 4.7, 6.3, and 8.4 mm (13) in the RL, SI, and AP directions with bony anatomy and 2, 4, and 5 mm (12) and 1.3, 1.2, and 1.2 mm (13) in these directions with online fiducial markers.
Because of the accuracy of kV planar imaging with fiducials, the necessity for CBCT has been questioned (12,13). The purpose of this study was to compare interfraction patient setup error in prostate radiotherapy using planar kV radiographs with patient anatomy, planar kV radiographs with fiducial markers, and CBCT with fiducial markers. With this frame of reference, the utility of CBCT was also assessed.
Materials and methods
Simulation
A total of 53 patients with prostate cancer undergoing radiation therapy were included in this study. All of the patients had three radioopaque fiducial markers implanted in their prostate 1-2 weeks prior to their treatment planning session, allowing for post-implantation seed migration. At the time of simulation, no special bowel preparation was performed and patients were instructed to have an empty bladder for both simulation and treatment. Patients were instructed to remove all clothing from the waist down to minimize displacement from the garments.
Immobilization and alignment consisted of supine positioning, indexed F roll under the head, hands folded on chest, and a leg-immobilization device encompassing the foot and separating the legs. Patients were straightened visually using the sagittal laser and prepared for scanning. A CT scan was performed using a Phillips Brilliance Big Bore scanner employing the pelvis protocol. Orthogonal scout films were obtained to check for pelvic rotation and tilt and patients were aligned accordingly. Patient were scanned from L1 vertebral body to below the lesser trochanter using 3 mm slices assuring all three fiducials were visible. Anterior, right and left lateral tattoos were placed at the isocenter coordinates. Following CT simulation and treatment planning, all patients were treated on a Varian iX® or Trilogy® linear accelerator equipped with on-board kV imaging capabilities.
Positioning and OBI
On days of treatment, patients were placed in the same position as the simulation and aligned to their three skin tattoos using treatment room sagittal and transverse lasers. An OBI (Varian Medical Systems) was utilized to take an orthogonal film pair. Visual confirmation of matching bony anatomy was performed to identify potential patient rotation, and, if discovered, the patient was realigned and re-imaged. Patient rotation was checked on the initial OBI films by examining the pelvic brim and obturator foramen for rotational displacement. If discovered, the patient was asked to raise and lower their pelvis and visually examined for rotation and straightness and setup again to tattoos and coordinates. This practice took care of all rotational misalignments we experienced.
During this process, implanted fiducial markers were also contoured on the anterior and lateral projections of the DRRs, and the kV images were overlaid onto them using the Varian® OBI matching program then manipulated in the inferior-superior, AP and left-right directions. Both automatic and manual matching was employed and implanted seeds were moved to the center of the DRR contour. If all three contours were misaligned, the patient would be repositioned and reimaged. When fiducials were matched, corresponding shifts were also sent electronically to the linear accelerator and the treatment table was moved to the desired location. All OBI film movements were reviewed and approved by the physician post treatment, or, pre-treatment if there was any question regarding patient or fiducial positioning.
Conebeam CT image acquisition
Twenty-five of the 53 patients in this study also underwent CBCT guided positioning in addition to orthogonal films. Twice a week, a 360˚ CBCT was performed immediately after fiducial matching shifts were carried out with the Varian kV OBI system, version 9. Scans were acquired using the pelvis protocol, 512 by 512 pixels and half-bowtie fan mount. After acquisition and reconstruction, the CBCT was matched in the transverse, sagittal and coronal planes to the fiducials in the original CT simulation scan and residual movements were performed and recorded for study purposes. All CBCT scans were reviewed and approved by the physician post treatment.
Treatment
Treatment was delivered by a Varian iX® or Trilogy® linear accelerator using 6 MV photons as prescribed in an intensity modulated radiation therapy treatment plan produced using Phillips Medical Systems, Pinnacle 3 (version 8.0 m) treatment planning system. Fractionation of 44 treatments to a total dose of 7,920 cGy was administered daily.
Statistical analysis
Descriptive statistics were calculated for shifts recorded using planar kV radiographs with bony anatomy and fiducial markers for all 53 prostate cancer patients. The Chi-square test was used to compare frequencies of shifts in longitudinal (SI), vertical (AP) and lateral (RL) planes. A “No shift” was defined as required movement of less than 1 mm (<0.1 cm). The difference in mean shifts in each direction was compared using a repeated measures ANOVA analysis using Proc MIXED in SAS 9.1 system (SAS Institute, Cary, NC, USA). The analysis allows modeling of variance-covariance matrix among repeated shifts recorded for each patient over the course of radiation treatment period.
In a subset of 25 patients who also underwent CBCT guided positioning residual set-up shifts were recorded and descriptive statistics and frequencies were calculated for each direction. Based on individual coordinate shifts, a net 3D residual shift magnitude vector “R” was calculated for each fraction according to following equation:
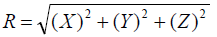
where R is the net residual 3D shift while X, Y and Z are residual shifts recorded in left-right, inferior-superior and AP directions, respectively. The frequencies of occurrence of residual 3D shifts were calculated for the CBCT subset. We regarded the residual 3D shift magnitude R as a useful figure of merit when considering the difference between planar imaging and CBCT for patient alignment. This is because PTV margins in conformal radiotherapy are generated in a 3D manner; therefore any discussion of the adequacy of setup precision is best framed in 3D terms as well. In addition, basing the analysis on the residual 3D shift R leads to an essential finding that is more compact and more easily communicated than is possible when each coordinate axis is examined separately. A two-sided hypothesis was used for all tests, and a probability value of less than 0.05 was considered statistically significant.
Results
Fifty-three patients underwent a total of 2,334 radiation treatment fractions. The mean shifts made by alignment with fiducial markers were significantly greater in the inferior and superior directions compared to alignment with bony anatomy alone (0.40 vs. 0.26 cm and 0.33 vs. 0.25 cm with P<0.0001, respectively). There were no statistically significant differences in mean shifts between bony anatomy and fiducial markers in different directions of vertical or lateral planes. A comparison of mean couch shifts (cm) recorded with kV radiographs using bony anatomy and fiducial markers is presented in Table 1.
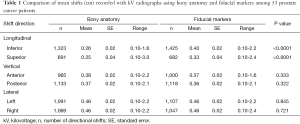
Full table
Bony anatomy vs. fiducials on kV images
Table 2 presents a comparison of the frequency with which couch shifts were required in those 53 patients following alignment to bony anatomy vs. fiducial markers. There was a significantly greater frequency of shifts in the inferior direction using fiducial markers compared to bony anatomy (61.1% vs. 56.7%, P<0.002). In addition, alignment to bony anatomy in the longitudinal plane more often resulted in no shift following initial patient setup when compared to fiducial marker alignment (13.7% vs. 9.7%, P<0.0001). There were no statistically significant differences in frequencies of shifts made in the vertical or lateral planes between these two image registration methods.
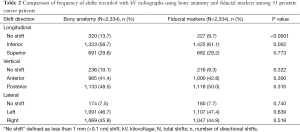
Full table
kV image vs. CBCT
In the subset of 25 patients (with 439 treatment fractions), once patient alignment and shifts were completed using kV images to fiducial markers, CBCT was done to match the fiducial markers to assess the need for any additional shifts. The residual shifts were recorded between the kV image-guided fiducial alignment and CBCT-guided fiducial alignment in Table 3. No residual shift was recorded in 66.5%, 52.4% and 57.9% of fractions for longitudinal, vertical and lateral planes, respectively. The mean residual shift was 0.17, 0.17, 0.16, 0.21, 0.15 and 0.15 cm in inferior, superior, anterior, posterior, left and right direction respectively. For net residual 3D shift R, the percentage of total treatment fractions requiring no shift, 0.1-0.3 cm shift, 0.3-0.5 cm shift, 0.5-0.7 cm shift, and greater than a 0.7 cm shift are presented in Figure 1. The majority (79%) of treatments required less than a 0.3 cm shifts or no shift using CBCT imaging after pre-alignment based on fiducials using kV imaging.

Full table
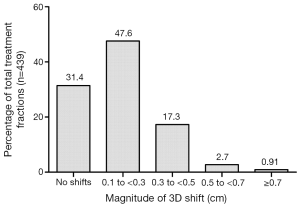
Treatment time
An analysis of randomly selected one-third of the study participants who received CBCTs along with OBI and their treatment, revealed patients having a CBCT were on the table about 14 min, whereas non-CBCT treatments were slightly over 8 min, which includes OBI seed matching. The difference was statistically significant (P<0.05) using t-test. These calculations were retrospectively performed using the documented beginning of the OBI film session and the time the last field was treated.
Discussion
Similar to the studies by Greer et al. and McNair et al., our data also suggest that kV imaging with alignment to fiducial markers is superior to alignment using bony anatomy alone (12,13). Mean shifts in the inferior and superior directions were significantly greater, whereas AP and left-right shifts did not differ. The larger daily shifts with fiducial markers are reflective of the fact that prostate is an internal organ that can move with respect to the skin markings and bony anatomy, requiring more shifts to account for the location variability. The reason for greater shifts required in the inferior-superior direction is unclear but review of literature suggests that this trend is not universal (12-15). One possible explanation is the daily prostate position shift caused by nearby organs such as the rectum and the bladder. Patients did not undergo daily bowel preparation prior to treatment and the amount of stool and gas in the rectum can certainly affect prostate positioning. In addition, patients were instructed to empty their bladder prior to simulation and treatment, but there is no effective way to standardize the post-void residual volume prior to simulation and treatment, especially considering many patients have enlarged prostates and voiding difficulties. A small study have investigated the bladder volume and prostate position changes using CBCT in patients who were instructed to keep their bladder full and found the bladder dimension to differ significantly in the inferior-superior direction but no significant shift in the target position (16). Further studies are needed to investigate the inter-fraction variability in size and shape of these surrounding organs that may explain our observation.
Fiducial-based imaging does not add any more time, radiation dose, or resources that are required for bony anatomy based imaging during a radiation treatment session. There have even been small studies that have reported on fiducial based imaging and its ability to reduce PTV margins (17). We believe that, in prostate radiotherapy, bony anatomy based alignment has inherent and significant limitations in localizing a soft tissue organ that is mobile with respect to the nearby bony structures, and it is therefore reasonable to replace this alignment technique with fiducial based imaging. According to the American College of Radiology (ACR) Appropriateness criteria, daily localization of the prostate with implanted markers is more appropriate than bony anatomy for external beam radiation therapy for patients with localized prostate cancer (18). Our results continue to support the mounting evidence of improved target localization afforded by fiducial based imaging. However, larger studies with follow-up data are needed to assess if the benefit of target localization with fiducial markers actually translates into better clinical outcomes.
Prostate localization using CBCT has the theoretical advantage of providing better soft tissue delineation and accurately localizing fiducial markers in 3D view, which cannot be accomplished by kV imaging. This is potentially important when the patient’s bladder and rectum sizes vary significantly on certain days of treatment. However, in the present study, we found only marginal benefit of CBCT for the alignment of implanted fiducials compared to kV radiographs. Residual mean shifts guided by CBCT were relatively small compared to kV imaging alignment (majority less than 0.3 cm). This small discrepancy in fiducial localization is to be expected due to the difference in target visualization between the two image registration methods and subtle patient motion while imaging is being obtained on treatment table and may not necessarily result in truly more accurate radiation delivery to the target organ. Shi et al. compared CBCT software-automated alignment to soft tissue markers vs. CBCT manual alignment to implanted fiducials, concluding that alignment to fiducials was the more accurate method (15). Barney et al. investigated CBCT-guided manual soft tissue alignment, comparing it to kV portal image-guided fiducial alignment (14). Although their data revealed that 60% of shift differences between these two methods were greater than 3.0 mm, the authors endorsed fiducial alignment based on CBCT-related increases in treatment times and the need for physician input for soft tissue alignment.
The greater length of time required for CBCT acquisition is likely not inconsequential, as studies have shown that the required margins for intrafractional motion increase with treatment time (19). In our study, the treatment time also significantly differed between the two groups, with CBCT patients taking on average 6 more minutes per treatment. Furthermore, CBCT increases overall patient exposure to ionizing radiation. Kan et al. reported that standard kV CBCT resulted in an effective dose of 22.7 mSv per scan to the pelvis and that if used daily could increase the secondary cancer risk by up to 2% to 4% (20).
Because of the propensity for the prostate to shift within the pelvis, an imaging localization modality that identifies the organ itself appears to be superior and can potentially result in smaller margins to account for errors. While fiducial markers are a good way to localize the prostate, their use is not universal in practice of radiation oncology. In addition, insertion of the markers is not without its own set of complications and this should be taken into consideration (11). When fiducials are not present, Palombarini et al. found that, compared to bone alignment with CBCT, soft tissue alignment resulted in larger degree of AP shifts due to random shifts in prostate position. The authors concluded that CBCT’s ability to see not only bony landmarks but also soft tissue gray scale allows for better localization of prostate in daily treatments and may reduce the PTV margins (21). This is especially important as the field starts moving towards more hypofractionated treatment, such as stereotactic body radiation therapy. In addition, CBCT imaging can offer the advantage of much better resolution of the pelvic structures, such as intra- or peri-prostatic calcifications, compared to plain films, which can be used as natural markers in select patients without the need to undergo invasive marker placement (22). Thus, CBCT may be more valuable in such cases where fiducials are not utilized. Large scale study directly comparing the use of fiducials in CBCT IGRT is warranted to further clarify which technique results in better outcome.
In interpreting the results of this study, several limitations must be addressed. Due to time constraints and resources available, CBCT was not universally done to all of the patients. This reduced the amount of data available in the CBCT group. As mentioned earlier, one of the advantages of CBCT is in its soft tissue resolution, allowing for assessment of not only prostate location but shape of surrounding anatomy, more frequent CBCT (daily with or without fiducial markers) could potentially provide more accurate treatment delivery. Another limitation of the study is its lack of clinical outcome with respect to disease control and toxicities. Future studies can potentially compare objective clinical findings and patient reported outcome to different target localization in order to assess the degree of benefit in more accurate treatments.
Conclusions
In conclusion, the results of this study indicate that daily prostate localization using fiducial markers in kV radiographs during radiation therapy for prostate cancer may offer better target localization compared to patient bony anatomy alone. The addition of CBCT to did not seem to provide significant additional benefit if fiducials are already in use but is associated with increased treatment time and radiation exposure to the patients. CBCT may be more useful in cases where fiducials have been lost and there are less than three present, or in patients who are medically unable to undergo fiducial placement.
Acknowledgements
All authors contributed to the design of the study. JC Ye was involved in all aspects of the study and co-wrote the paper. MM Qureshi performed the data analysis and was involved in interpretation of results along with assisting in writing the paper. P Clancy and LN Dise were involved in carrying out the treatment and collection of data. J Willins designed the method to calculate 3D shift and was involved in editing the manuscript. AE Hirsch supervised this study and was involved in all aspects of the study from study design to data interpretation and co-wrote paper.
Footnote
Conflicts of Interest: Presented in part at the 52nd Annual Meeting of the American Society for Radiation Oncology, Oct 31-Nov 4, 2010, San Diego, CA, USA.
References
- Xing L, Thorndyke B, Schreibmann E, Yang Y, Li TF, Kim GY, Luxton G, Koong A. Overview of image-guided radiation therapy. Med Dosim 2006;31:91-112. [PubMed]
- Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys 2001;51:880-914. [PubMed]
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, Shipley WU. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233-9. [PubMed]
- Lawson JD, Fox T, Elder E, Nowlan A, Davis L, Keller J, Crocker I. Early clinical experience with kilovoltage image-guided radiation therapy for interfraction motion management. Med Dosim 2008;33:268-74. [PubMed]
- Huntzinger C, Munro P, Johnson S, Miettinen M, Zankowski C, Ahlstrom G, Glettig R, Filliberti R, Kaissl W, Kamber M, Amstutz M, Bouchet L, Klebanov D, Mostafavi H, Stark R. Dynamic targeting image-guided radiotherapy. Med Dosim 2006;31:113-25. [PubMed]
- Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, Bissonnette JP, Gospodarowicz M, Warde P, Catton CN, Jaffray DA. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007;67:942-53. [PubMed]
- Verellen D, De Ridder M, Tournel K, Duchateau M, Reynders T, Gevaert T, Linthout N, Storme G. An overview of volumetric imaging technologies and their quality assurance for IGRT. Acta Oncol 2008;47:1271-8. [PubMed]
- Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2002;53:1337-49. [PubMed]
- Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys 2009;73:610-7. [PubMed]
- Scarbrough TJ, Golden NM, Ting JY, Fuller CD, Wong A, Kupelian PA, Thomas CR Jr. Comparison of ultrasound and implanted seed marker prostate localization methods: Implications for image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:378-87. [PubMed]
- Fuller CD, Scarbrough TJ. Fiducial Markers in Image-guided Radiotherapy of the Prostate. US Oncological Disease 2006;1:75-9.
- Greer PB, Dahl K, Ebert MA, Wratten C, White M, Denham JW. Comparison of prostate set-up accuracy and margins with off-line bony anatomy corrections and online implanted fiducial-based corrections. J Med Imaging Radiat Oncol 2008;52:511-6. [PubMed]
- McNair HA, Hansen VN, Parker CC, Evans PM, Norman A, Miles E, Harris EJ, Del-Acroix L, Smith E, Keane R, Khoo VS, Thompson AC, Dearnaley DP. A comparison of the use of bony anatomy and internal markers for offline verification and an evaluation of the potential benefit of online and offline verification protocols for prostate radiotherapy. Int J Radiat Oncol Biol Phys 2008;71:41-50. [PubMed]
- Barney BM, Lee RJ, Handrahan D, Welsh KT, Cook JT, Sause WT. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011;80:301-5. [PubMed]
- Shi W, Li JG, Zlotecki RA, Yeung A, Newlin H, Palta J, Liu C, Chvetsov AV, Olivier K. Evaluation of kV cone-beam ct performance for prostate IGRT: a comparison of automatic grey-value alignment to implanted fiducial-marker alignment. Am J Clin Oncol 2011;34:16-21. [PubMed]
- Tsai CL, Wu JK, Wang CW, Hsu FM, Lai MK, Cheng JC. Using cone-beam computed tomography to evaluate the impact of bladder filling status on target position in prostate radiotherapy. Strahlenther Onkol 2009;185:588-95. [PubMed]
- Skarsgard D, Cadman P, El-Gayed A, Pearcey R, Tai P, Pervez N, Wu J. Planning target volume margins for prostate radiotherapy using daily electronic portal imaging and implanted fiducial markers. Radiat Oncol 2010;5:52. [PubMed]
- Abdel-Wahab M, Mahmoud O, Merrick G, Hsu IC, Arterbery VE, Ciezki JP, Frank SJ, Mohler JL, Moran BJ, Rosenthal SA, Rossi CJ Jr, Yamada Y. ACR Appropriateness Criteria® external-beam radiation therapy treatment planning for clinically localized prostate cancer. J Am Coll Radiol 2012;9:233-8. [PubMed]
- Kotte AN, Hofman P, Lagendijk JJ, van Vulpen M, van der Heide UA. Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys 2007;69:419-25. [PubMed]
- Kan MW, Leung LH, Wong W, Lam N. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2008;70:272-9. [PubMed]
- Palombarini M, Mengoli S, Fantazzini P, Cadioli C, Degli Esposti C, Frezza GP. Analysis of inter-fraction setup errors and organ motion by daily kilovoltage cone beam computed tomography in intensity modulated radiotherapy of prostate cancer. Radiat Oncol 2012;7:56. [PubMed]
- Hanna SA, Neves-Junior WF, Marta GN, Haddad CM, da Silva JL. Role of intra- or periprostatic calcifications in image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:1208-16. [PubMed]


