Assessment of risk factors of pediatric urolithiasis in Egypt
Introduction
Renal stone disease is a significant medical problem, which has experienced an increasing incidence worldwide (1,2). This may be due to the increased awareness of the entity or to the routine use of ultrasonography in children presenting with specific or nonspecific symptoms for urolithiasis. Although epidemiological data from some parts of the world are unclear, it is estimated that the incidence of stone disease in children is about 2-3% (3).
Risk factors for urolithiasis in children are multifactorial, including genetic inheritance, nutrition, metabolic abnormalities, environmental factors and stone-inducing medicines (4). However, as the clinical and metabolic patterns of urolithiasis have changed over the years (1,5), this tendency may become more prevalent and thus necessitates an evaluation of the potential recent risk factors.
Our perceptive of the Pathophysiology of calculus disease in children has grown immensely. As most children with stone disease have an underlying metabolic abnormality (3), it is necessary that these children should be cautiously evaluated so that the etiology of their disorder can be obtained. In this way, future stone formation and/or reformation may be prohibited in the pediatric population, limiting the morbidity of the disease.
Unlike other pathologies, the incidence and nature of pediatric urolithiasis differ significantly from country to country (6). Unfortunately, most population-studies on pediatric urolithiasis were from Europe or North America, and there was deficient data on risk factors for urolithiasis among Egyptian children. The main objective of the present study was to investigate the clinical characteristics and the most critical risk factors that contribute to stone formation in Egyptian children.
Subjects and methods
This prospective study was carried out at the outpatient clinics of Cairo university children’s hospital as well as October 6 University hospital, between November 2008 and March 2012. Any child between the age of one and fourteen years, presenting with gross hematuria, renal colic or recurrent urinary tract infections, was included in the study. In this group, stones were documented through plain X-ray and/or renal ultrasound, and in selected cases by intravenous urography. Children with documented or suspected urolithiasis who were referred to our hospital were also included in the study. The following exclusion criteria were adopted; Patients with congenital abnormalities, metabolic/intestinal disorders, endocrinal diseases and renal or hepatic insults.
One hundred and fifty children out of two hundred and five, satisfied the study criteria and were enrolled in the study. As a control, all evaluation procedures were performed and interpreted in 30 healthy children.
A detailed medical history, together with dietary habits and family background, were obtained from the parents as well as a physical examination, including Growth percentiles was performed. Then, all patients underwent laboratory investigations including: complete urine analysis and culture and sensitivity tests, urine collection in 24-h to quantify urinary volume, pH, and urinary calcium, uric acid, magnesium, creatinine, oxalate and citrate. Blood samples were obtained to estimate necessary biochemical parameters as (serum creatinine, calcium, phosphorus, uric acid level, and alkaline phosphatase and electrolyte levels, in addition to pH and pCO2 values).
Urine samples were collected in standard plastic bottles containing 10 mL 6-N hydrochloric acid. All study samples were acidified and alkalinized for measurements of calcium oxalate and urate respectively. Samples were cooled during the collection period and frozen at –20 °C until the examination. Calcium, creatinine, uric acid and phosphorus levels in the serum and urine samples were measured using a specific technique (7), urinary citrate was detected with the citrate lyase technique (8) and urinary oxalate was detected using the enzymatic scheme (9). Metabolic diagnosis consisted of hypercalciuria >4 mg/kg/24 hour, hypocitraturia <220 mg/day), hyperoxaluria >50 mg/1.73 m2/24 hours), and hyperuricosuria >10.7 mg/kg/day). Normal urinary constituents values had been estimated as <1.2 mg/kg for magnesium and >15 mg/kg for phosphate (10).
Following laboratory evaluation, all children underwent a thorough radio-sonographic investigation of the abdomen and the pelvis to evaluate (possible anatomic abnormalities of the genitourinary tract, the presence and the degree of backpressure caused by the stone(s), the existence of other stones and the presence of nephrocalcinosis). Intravenous pyelography and/or renal scintigraphy, CT and cystourethrography were used when required.
Patients who did not improve with conservative treatment were managed by different treatment modalities, including extracorporeal shock-wave lithotripsy (ESWL), ureteroscopy and open surgical management. However, ESWL was the preferable choice for most of the patients.
All stone samples obtained by means of open operation, ESWL, endoscopic procedures, or spontaneous passage were analyzed by spectrophotometry.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using Student t test for independent samples in comparing 2 groups, and one way analysis of variance (ANOVA) test with multiple 2-group comparisons in comparing more than 2 groups. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency was less than 5. Correlation between various variables was done using Spearman rank correlation equation. P values less than 0.05 was considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
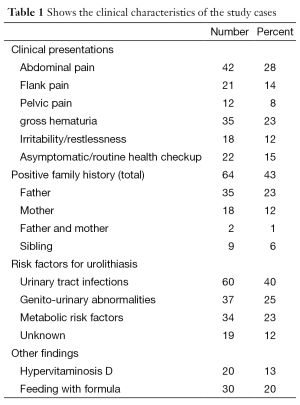
In the present study, the patient group consisted of 100 boys and 50 girls (ratio 2:1). The mean age at diagnosis of stone disease was 3.5 years (range, 1 year to 14 years). The mean follow-up duration was 33.1 months. The presenting symptoms and criteria of patients with stones are shown in (Table 1).
Full table
The main clinical presentations of our patients were abdominal pain in 42 children (28%), flank pain in 21 children (14%), and pelvic pain in 12 children (8%). Gross hematuria was the second most common presentation; 35 patients (23%) followed by irritability/restlessness in 18 infants (12%). However 15% of the study cases were diagnosed during routine checkups.
Evaluation of the detailed histories provided by the parents revealed a history of stone passage before referral in 23 children (15%). A positive family history was noted in 64 children (43%).
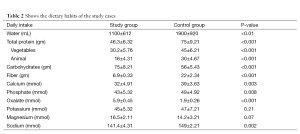
The dietary habits of the children were carefully investigated and comparative assessment of the content of the food consumed, confirmed diminished intake of water, total protein (especially of animal origin) and food with a high fiber content. Additionally, increased intake of starchy foods and foods with elevated oxalate content were observed in our study cases (Table 2).
Full table
A total of 33/150 (22%) patients had recurrent urinary stones, 16 (11%) of whom had a history of at least one previous surgical procedure for stone disease. 12 (8%) children had undergone a previous endoscopic intervention and 4 (3%) had undergone open surgery.
Evaluation of growth percentiles, using growth curves defined for Egyptian children by Ghalli et al. (11), revealed a failure to thrive in a considerable proportion of the patients, where 40 children (27%) were below the 3rd percentile.
Regarding stone localization, the majority of the patients had stones localized in the upper urinary tract. Stones were localized in the renal calyces in 72 patients (48%) and in the renal pelvis in 52 patients (35%). Of the 26 patients with stones localized in the extrarenal region, 16 patients (12%) presented with stones in the ureters, 6 patients (4%) in the bladder, and 4 patients (3%) in the posterior urethra.
A total of 37 (25%) patients had bilateral stone disease and 113 (75%) patients had unilateral stone disease. Multiple stones were found in 12 patients (8%). The mean stone diameter was 2.7 cm (range, 0.2-3.3 cm)
Concerning the possible predisposing factors, 60 patients (40%) had UTI at the time of diagnosis and were treated with appropriate medications. A history of recurrent UTI was present in 70% of these children. Associated genito-urinary abnormalities were detected in 38 children (25%), including vesico-uretheral refux in (18/38), primary obstructive uropathies such as ureteropelvic junction stenosis (10/38), uretero-vesical stenosis (8/38), and horseshoe kidney (2/18).
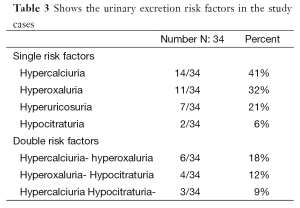
Evaluation of metabolic risk factors in 24-h urine samples revealed 34 children (23%) with a single or multiple risk factors with hypercalciuria and/or hyperoxaluria being the commonest metabolic abnormalities reported in our cases (Table 3). Table 4 shows urinary levels of certain risk factors in the patient and control groups.
Full table

Full table
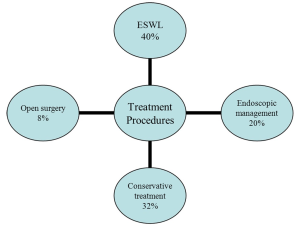
Concerning the treatment procedures performed, stones were managed with ESWL in 60 patients (40%), endoscopic procedures (simple forceps and/or basket extraction) were used in 40 children (20%) and 12 children (8%) underwent an open surgical treatment. The remaining 48 children (32%) were followed up with conservative treatment and the majority of them passed the stones spontaneously (Figure 1). No major complications related to treatments were found.
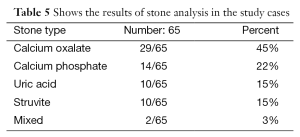
Stone samples were available for analysis in 65 patients and the majority (45%) of these children had calcium oxalate stones (Table 5). There were significant correlations between stone size and urinary citrate excretion (P<0.05) and between the presence of UTI and urinary phosphate excretion (P=0.032).
Full table
Discussion
Pediatric urolithiasis is an important medical problem, which has seen an increasing incidence in developing countries. The number of new patients referred to our clinics for urolithiasis per year has increased in the last few years. Van Dervoort et al. (12) documented a similar trend in that the incidence of urolithiasis in the pediatric population had augmented nearly five folds during the last decade in USA. The present study was conducted to investigate the clinical characteristics and the most crucial risk factors that contribute to stone formation in Egyptian children
There are few articles on urolithiasis in children (13-17). The majority of these articles have reported a male predominance, with the male to female ratio varying from 1:1 to 4:1, This is in agreement with our study where the male-to-female ratio was 2:1, However, on the contrary, Edvardsson et al. (18) studied 26 patients and found females to be at a higher risk being anatomically more susceptible to infections than male.
A positive family history for urolithiasis was present in 43% of our patients. Urolithiasis among family members may indicate a relative risk for stone formation. Rates of a positive family history in children with urolithiasis were as high as 54% in other studies conducted in Turkey (3). Rates as high as 78.7% were reported in Argentina, along with comparable numbers (69-78%) in Italy (19). The rate of intermarriage in our country is very high; this may be a factor in the genetic tendency towards urolithiasis in our study group. However, it is important to determine that familial recurrence does not necessarily imply an inherited genetic cause. Shared environmental factors and common dietary habits can contribute to familial predisposition.
According to the literature, the major symptoms of urolithiasis in childhood are abdominal colic and gross hematuria (20,21). Similarly, in the current study abdominal pain followed by gross hematuria were the most common presentations. 15% of our cases were asymptomatic, diagnosed during routine health checkup, whereas Baştuğ et al. (22) studied 178 infants and reported that 13% of them were asymptomatic.
In the present study the importance of UTI as a crucial risk factor in stone formation was demonstrated, 40% of the patients had UTI at the time of diagnosis, 70% of them had history of recurrent UTI. This is in agreement with studies of Erbagci et al. (3) and Sternberg et al. (23). Our findings also showed that stones can predispose to UTI; conversely, UTI itself may be important in the formation of stones. However, only 15% of our cases had infectious stones, which could be attributed to the fact that urinary tract infections may be the primary cause of calculus formation, or may occur because of other metabolic causes (24). When urinary infections are the primary cause, it produces calculi composed of struvite (magnesium-ammonium-phosphate) and carbonate apatite. These stones form as a result of the bacterial enzyme urease, which hydrolyzes urinary urea to ammonia and carbon dioxide. This produces an alkaline urinary environment and favors the formation of struvite calculi. Organisms known to produce urease include Proteus, Klebsiella, and Pseudomonas (24).
Urinary tract infections in infancy presented with failure to thrive in 30% of cases (25). In the current study, 27% of cases were below the 3rd centile and had failure to thrive. This high figure is explained by the high prevalence of UTI among our patients. Moreover, history of overdose of vitamin D administration was reported in twenty infants, all of which had rickets and poor nutritional history.
In contrast to ain vivodults, pediatric urolithiasis is more often associated with underlying metabolic abnormalities (26,27). In the literature, the frequency of metabolic anomalies varies from 15% to 90% of cases (14). This huge variation can be explained mainly by the lack of agreement on the importance of urinary biology in children and particularly in infants (22). In the present study, 23% of children demonstrated with metabolic disorders, the commonest abnormalities were hypercalciuria and hyperoxaluria. Similar findings were observed by Guven et al. (15) and Alpay et al. (14) On the contrary, Baştuğ et al. (22) found hyperuricosuria to be the most common metabolic risk factor in their series.
In the present study there was a history of overdose of vitamin D administration in 20 infants (13%) and feeding with formula in 30 (20%). Hypervitaminosis D was identified as a possible cause of hypercalciuria, and it sometimes contributed to the misuse of high doses of vitamin D in infants. In addition, infant formulas are rich in calcium, phosphorus, oxalate and sulfate and have adequate amounts of vitamin B6, vitamin D and ascorbic acid (28). Therefore, the use of prophylactic doses of vitamin D in formula-fed infants may result in infants receiving more vitamin D than necessary. Vitamin D levels were not measured in our patients. Although, the increased incidence of infantile stone disease in the recent years may be associated with an increased rate of diagnosis because ultrasound is more frequently used, it may also be related to the use of infant formulas containing vitamins and inappropriate doses of vitamin D.
A urinary tract anomaly has long been considered as a predisposing condition for the formation of calculi, with associated urinary stasis which is considered to promote crystal retention and stone formation. It has been reported that about 10-19% of children with urolithiasis have an underlying malformation of the urinary tract (18,23). In the present study, 25% of our patients had urologic abnormalities, with vesico-uretheral refux being the most common abnormality.
Evaluation of stone localization in our group demonstrated that the majority of the stones were located in the upper urinary tract, (72% in the renal calyces and 35% in the renal pelvis) which is consistent with the results of many recent studies (12,14). Only 4% of the children had bladder stones and the incidence of such stones tended to decrease with increasing age, as reported in an earlier study by Tellaloğlu et al. (29).
There are minor differences in stone composition among the reported cases in children worldwide, with most series reflecting a similar stone composition to that seen in adults. Most children have stones composed of calcium oxalate or calcium phosphate with struvite, uric acid, and less often cystine stones (3).
Stone samples were available for analysis in 65 patients, (45%) of these children had calcium oxalate stones.
In children, the rate of recurrence of stones ranges widely from 3.6% to 67% and observed to be highest in children with metabolic abnormalities (3,12,22,27). These findings are comparable to our results, where among the 33 (22%) patient who developed recurrent urinary stones, 23 cases had metabolic risk factors.
Conclusions
Because of the multifactorial causes of stones in children (metabolic, anatomic and/or recurrent UTI), treatment can only be achievable when combined with appropriate prophylaxis to prevent recurrence. The objectives of stone management in children should be complete stone clearance, avoidance of stone recurrence and re-growth, preservation of renal function, proper management of UTIs, correction of anatomic anomalies and adjustment of metabolic disorders.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol 2010;25:49-59. [PubMed]
- Mohamed J, Riadh M, Abdellatif N. Urolithiasis in infants. Pediatr Surg Int 2007;23:295-9. [PubMed]
- Erbagci A, Erbagci AB, Yilmaz M, et al. Pediatric urolithiasis--evaluation of risk factors in 95 children. Scand J Urol Nephrol 2003;37:129-33. [PubMed]
- Milliner DS. Urolithiasis. In: Avner ED, Harman WE, Niaudet P, et al. eds. Pediatric nephrology. 6th edn. Berlin: Springer, 2009:1405-24.
- Sarkissian A, Babloyan A, Arikyants N, et al. Pediatric urolithiasis in Armenia: a study of 198 patients observed from 1991 to 1999. Pediatr Nephrol 2001;16:728-32. [PubMed]
- Tekin A, Tekgul S, Atsu N, et al. A study of the etiology of idiopathic calcium urolithiasis in children: hypocitruria is the most important risk factor. J Urol 2000;164:162-5. [PubMed]
- Gosling P. Analytical reviews in clinical biochemistry: calcium measurement. Ann Clin Biochem 1986;23:146-56. [PubMed]
- Welshman SG, McCambridge H. The estimation of citrate in serum and urine using a citrate lyase technique. Clin Chim Acta 1973;46:243-6. [PubMed]
- Chiriboga J. Some properties of an oxalic oxidase purified from barley seedlings. Biochem Biophys Res Commun 1963;11:277-82.
- Kroovand RL. Pediatric urolithiasis. Urol Clin North Am 1997;24:173-84. [PubMed]
- Ghalli I, Salah N, Hussien F, et al. In: Proceedings of the 1st National Congress for Egyptian Growth Curves, Cairo University, 11 December 2003.
- VanDervoort K, Wiesen J, Frank R, et al. Urolithiasis in pediatric patients: a single center study of incidence, clinical presentation and outcome. J Urol 2007;177:2300-5. [PubMed]
- Mohamed J, Riadh M, Abdellatif N. Urolithiasis in infants. Pediatr Surg Int 2007;23:295-9. [PubMed]
- Alpay H, Ozen A, Gokce I, et al. Clinical and metabolic features of urolithiasis and microlithiasis in children. Pediatr Nephrol 2009;24:2203-9. [PubMed]
- Güven AG, Koyun M, Baysal YE, et al. Urolithiasis in the first year of life. Pediatr Nephrol 2010;25:129-34. [PubMed]
- Dursun I, Poyrazoglu HM, Dusunsel R, et al. Pediatric urolithiasis: an 8-year experience of single centre. Int Urol Nephrol 2008;40:3-9. [PubMed]
- Zvara V. Diagnostic and treatment of urolithiasis in infants and children. Urol Nephrol 1961;1:243-250.
- Edvardsson V, Elidottir H, Indridason OS, et al. High incidence of kidney stones in Icelandic children. Pediatr Nephrol 2005;20:940-4. [PubMed]
- Spivacow FR, Negri AL, del Valle EE, et al. Metabolic risk factors in children with kidney stone disease. Pediatr Nephrol 2008;23:1129-33. [PubMed]
- Dursun I, Poyrazoglu HM, Dusunsel R, et al. Pediatric urolithiasis: an 8-year experience of single centre. Int Urol Nephrol 2008;40:3-9. [PubMed]
- Lottmann HB, Archambaud F, Traxer O, et al. The efficacy and parenchymal consequences of extracorporeal shock wave lithotripsy in infants. BJU Int 2000;85:311-5. [PubMed]
- Baştuğ F, Gündüz Z, Tülpar S, et al. Urolithiasis in infants: evaluation of risk factors. World J Urol 2013;31:1117-22. [PubMed]
- Sternberg K, Greenfield SP, Williot P, et al. Pediatric stone disease: an evolving experience. J Urol 2005;174:1711-4; discussion 1714.
- Thomas S, Stapleton FB. Pediatric urolithiasis: diagnosis and management. In: Gonzales E, Bauer SB, eds. Pediatric urology practice. Philadelphia (PA): Lippincott-Raven Press, 1999:607-21.
- Qureshi AM. Clinical presentation of urinary tract infection among children at Ayub Teaching Hospital, Abbottabad. J Ayub Med Coll Abbottabad 2005;17:79-81. [PubMed]
- Ali SH, Rifat UN. Etiological and clinical patterns of childhood urolithiasis in Iraq. Pediatr Nephrol 2005;20:1453-7. [PubMed]
- Cameron MA, Sakhaee K, Moe OW. Nephrolithiasis in children. Pediatr Nephrol 2005;20:1587-92. [PubMed]
- Hoppe B, Roth B, Bauerfeld C, et al. Oxalate, citrate, and sulfate concentration in human milk compared with formula preparations: influence on urinary anion excretion. J Pediatr Gastroenterol Nutr 1998;27:383-6. [PubMed]
- Tellaloğlu S, Ander H. Stones in children. Turk J Pediatr 1984;26:51-60. [PubMed]