Translating insights of AR signaling from mouse models
Androgen receptor (AR) signaling in prostate cancer
The AR gene located on the X chromosome, encodes for a 110 kDa nuclear hormone receptor protein that mediates the transcription of target genes. Androgens bind to AR (ligand binding domain) and orchestrate a transcriptional program mediating cell growth, proliferation, differentiation, and homeostasis of androgen dependent cells. AR signaling is crucial for the development and maintenance of male reproductive organs including the prostate gland.
Huggins and Hodges first demonstrate that prostate cancer was dependent on androgen signaling by observing disease regression in men with prostate cancer following bilateral orchiectomy (1). Since that time, androgen deprivation therapy has been the treatment of choice for patients with locally advanced and metastatic prostate cancer. Despite the initial response to androgen deprivation therapy in patients with metastatic prostate cancer, the majority of patients develop progressive disease (castrate resistant prostate cancer), which recently has been shown to still be dependent on persistent androgen signaling (2-4). Through the identification of the molecular mechanisms promoting AR signaling in the setting of castration (AR amplification, AR over expression, AR mutation, peripheral androgen production) a number of novel therapies (enzalutamide, zytiga) have successfully been developed and have been shown to improve survival in patients with castrate resistant disease (5-8). However, even with the advances of the second-generation AR pathway inhibitors, the majority of patients still suffer disease progression with active AR signaling, highlighting the importance of the need to identify the mechanisms of resistant disease and further explore alternative pathways that may promote cell death alone or in combination with AR targeted therapies.
In the current manuscript we will review the knowledge of AR signaling gained through pre-clinical mouse models with an emphasis on how this has translated to our clinical understanding and management of prostate cancer.
Murine prostate response to castration
Unlike humans, mice do not express the enzyme required for the production of adrenal androgens and hence surgical castration results in complete hypogonadism. In the wild-type mice, surgical castration induces a wave of luminal epithelial cell apoptosis over an initial 3 days period followed by prostate gland atrophy (9). Despite this tremendous decrease in prostate gland size and luminal cell death, non-androgen dependent basal epithelial cells and a small percentage of luminal cells persist in the prostate. Studies have demonstrated that following castration the add back of testosterone is capable of reconstituting prostate gland size and cellular differentiation (9). Through studies of castration and regeneration, several groups have isolated progenitor cells from the prostate with the capability of giving rise to basal and luminal epithelial cells (9,10). These castrate resistant progenitor cells have been identified through lineage tracing to be present in the basal cell and luminal cell compartments. While linage different progenitor cells have been observed (basal and luminal) they share common features with regards to being castrate resistant, the ability to reconstitute basal and differentiated luminal cells, and following oncogenic insult promote a prostate cancer phenotype (9,10). This work has led to an improved but not complete understanding of the cell of origin of prostate cancer and castrate resistant phenotypes.
AR knock-out mice
To further explore the role of AR signaling in the prostate, a series of genetically engineered mouse (GEM) models using the Cre-Lox system have been developed. The models generate knock-out the AR gene leading to diminished transcript and absent protein production in a Cre inducible fashion, where the expression of Cre can be driven by cell/tissue specific promoters (11,12). This technology allows the AR gene to be knocked-out in a cell specific fashion, with the caveat that Cre expression can be leaky and heterogeneous.
Ubiquitous knock-out of AR using an actin regulated Cre model revealed that the male progeny had ambiguous external genitalia with a hypospadiac microphallus, testicular atrophy, and agenesis of the vas deferens, epididymis, seminal vesicles and prostate gland (12). This work confirmed the critical role of AR in male reproductive development. Furthermore, through this work, androgen signaling was demonstrated to play a critical role in the immune and skeletal organ systems. Using tissue specific Cre driven models, several investigators have evaluated the role of AR in various prostatic compartments (luminal, stomal) to evaluate physiologic and oncogenic prostate biology. Using the probasin promoter to drive Cre expression and knock-out AR in the luminal epithelial cells of the prostate post-pubertal resulted in a phenotype of basal epithelial cell hyperplasia without differentiation to a luminal cell phenotype (13). This data confirmed the role of androgen signaling in promoting luminal cell differentiation. Knock-out of AR in the prostate stroma smooth muscle component, using the transgelin promoter to drive Cre expression, resulted in a relatively normal prostate epithelial phenotype with a slight reduction in luminal cell infolding (14). The most striking phenotype observed in this mouse model was a reduced stromal cell proliferation which was associated with a decrease in IGF-1 levels and signaling. Collectively these GEM models have allowed us to evaluate the inhibition of AR in a cell and tissue specific manner to elucidate the role of AR signaling in a cell specific context that could not be obtained from androgen deprivation therapies alone.
AR inhibition in GEM models of prostate cancer
Inhibition of the AR axis is the mainstay of treatment for locally advanced and metastatic prostate cancer. Genomic profiling studies in prostate cancer have revealed that loss of the tumor suppressors PTEN and TP53, amplification of MYC, and genomic rearrangements involving ERG are amongst the most common alterations present in prostate cancer. PTEN loss is reported to occur in approximately 50% of metastatic prostate cancer specimens and is significantly associated with concomitant loss of TP53 and ERG genomic rearrangements. Based on these findings, several GEM models of prostate cancer have evaluated the biologic role of these oncogenic events in prostate tumorigenesis leading to the development of mouse models that spontaneously develop prostate cancer (15-18). Using these models, several groups have evaluated the impact of specific genetic alterations on response to AR pathway inhibition in these GEM models to determine molecular predictors of response and resistance.
The Pb-MYC model developed by Sawyers and colleagues has been shown to display sensitivity to androgen deprivation by surgical castration at early time points while aged mice reveal castrate resistant disease that is still sensitive to combined androgen blockade (surgical castration + enzalutamide, an AR antagonist) (15,19). The Pten loss series of mouse models developed by the Pandolfi lab demonstrate castrate and AR inhibitor resistant phenotypes, despite significant down regulation of AR target gene expression (19). This data is further reinforced through work by Mullholland et al., where epithelial knock-out of AR did not promote tumor regression in a GEM model of Pten loss (20). These studies highlight that loss of PTEN in prostate cancer is associated with tumor cell survival independent on AR pathway activation. Ongoing studies are evaluating the ability of loss of PTEN to serve as a predictive biomarker for response to AR inhibition in patients with advanced metastatic prostate cancer.
Genetic determinants of AR signaling
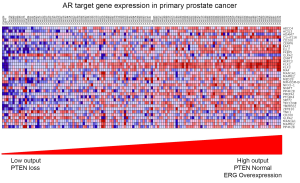
Molecular profiling studies in GEM models of prostate cancer have improved our understanding of the pathways regulating AR activity and downstream target gene expression. By analyzing the prostate transcriptome profiles of wild-type mice pre- and post-castration, Carver and colleagues developed a murine AR responsive gene signature (19). This gene signature allows investigators to determine the degree of AR activity across differing genetic context or AR targeted therapy in the mouse prostate. Based on this it has been demonstrated by several investigators that loss of the tumor suppressor Pten, resulting in activation of PI3K signaling, is associated with reduced AR target gene activity and repressed AR output (19,20). This may explain in part the resistance of AR targeted therapies in the setting of Pten loss as these tumors are inherently less dependent on AR signaling. Importantly, castration in the setting of AR pathway inhibition further suppresses the murine AR responsive gene signature indicating that this pathway is still functional. Additionally, inhibition of the PI3K pathway in the setting of Pten loss resulted in increased AR target gene expression. Through a series of experiments if has been established that the PI3K and AR pathways Based on these findings, studies have demonstrated that AR target gene activity in primary and metastatic prostate cancer specimens is quite variable and also dependent on genetic context with tumors displaying loss of PTEN having reduced AR target gene expression compared to tumors with a normal PTEN status (Figure 1) (19).
Furthermore, Chen and colleagues have recently demonstrate that genomic rearrangements of ERG, which are presented in approximately 60% of PTEN loss tumors, can partially restore AR target gene expression in the setting of Pten loss (21). Chromatin IP experiments in GEM models demonstrated that over-expression of ERG dramatically increased the number of AR binding sites, thus priming the chromatin for AR binding. These findings were also observed in patient derived metastatic prostate cancer specimens. Collectively this data has improved our understanding of the role that molecular alterations outside of AR may play in regulating AR target gene activity. It is becoming increasingly appreciated that AR activity is not just present or absent, but present across a wide spectrum of activity in both murine and human prostate cancer. This understanding will have a significant impact for predicting sensitivity to and quantifying the degree of AR pathway inhibition in prostate cancer.
Conclusions
The AR pathway plays a critical role in prostate cancer biology and thus targeting AR for inhibition is the mainstay of treatment for locally advanced and metastatic prostate cancer. Through mouse modeling work we have gained an improved understanding of genetic determinants of resistance to AR targeted therapies and an improved understanding of how oncogenic pathways regulate AR target gene expression. Collectively this work may allow for better prediction of which patients will respond long-term to androgen pathway inhibition and in which patients combination therapies may be required to optimize outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. [PubMed]
- Smaletz O, Scher HI. Outcome predictions for patients with metastatic prostate cancer. Semin Urol Oncol 2002;20:155-63. [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [PubMed]
- Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol 2003;21:2673-8. [PubMed]
- Edwards J, Krishna NS, Grigor KM, et al. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer 2003;89:552-6. [PubMed]
- Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol 2004;164:217-27. [PubMed]
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [PubMed]
- Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009;461:495-500. [PubMed]
- Wang ZA, Mitrofanova A, Bergren SK, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol 2013;15:274-83. [PubMed]
- De Gendt K, Swinnen JV, Saunders PT, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 2004;101:1327-32. [PubMed]
- Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A 2002;99:13498-503. [PubMed]
- Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 2001;101:61-9. [PubMed]
- Yu S, Zhang C, Lin CC, et al. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate 2011;71:517-24. [PubMed]
- Ellwood-Yen K, Graeber TG, Wongvipat J, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003;4:223-38. [PubMed]
- Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol 2003;1:E59. [PubMed]
- Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 2009;41:619-24. [PubMed]
- Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005;436:725-30. [PubMed]
- Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011;19:575-86. [PubMed]
- Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011;19:792-804. [PubMed]
- Chen Y, Chi P, Rockowitz S, et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat Med 2013;19:1023-9. [PubMed]