Steroid hormone synthetic pathways in prostate cancer
Presence of intratumoral androgens despite castration
The efficacy of androgen deprivation therapy (ADT) is routinely based on achieving castrate levels of serum T, defined as <20 ng/dL. However, tissue androgen levels in the setting of benign prostatic hyperplasia (BPH), primary prostate tumors, locally recurrent prostate cancer (PCa), or metastatic castration-resistant prostate cancer (CRPC) have consistently demonstrated that castration does not eliminate androgens from the prostate tumor microenvironment.
Geller et al. examined prostatic DHT levels by radioimmunoassay (RIA) and demonstrated that castration by orchiectomy (or megace plus DES) reduced prostatic DHT levels by 75-80% to 1 ng/g in some but not all patients. Epithelial and stromal cell protein synthesis was strongly correlated with tissue DHT levels, and prostatic DHT levels were further reduced when castration was combined with adrenal androgen blockade by ketoconazole (1-6), suggesting the goal of therapy should be to decrease prostatic DHT to as low as possible, a concept similarly framed in early studies by Labrie (7).
Incomplete suppression of prostate tissue androgens by castration has been subsequently confirmed in numerous studies of short and long term castration therapy (8). Treatment of BPH patients for 3 months with an LHRH agonist decreased intraprostatic T levels by 75%, to about 0.1 ng/g, and DHT levels by 90%, to 0.48 ng/g (9). In men with PCa 6 months of neoadjuvant ADT with castration and flutamide reduced prostatic DHT levels by 75% to about 1.35 ng/g (10). Notably, tumor differentiation based on Gleason grading has been correlated with change in tissue DHT, with an 85% decrease measured in Gleason 6 cancers, but only a 60% decrement in Gleason 7-10 tumors (11). This finding indicates that tumor type-specific changes in androgen metabolism may impact responses to systemic T suppression.
Residual androgens have also been demonstrated in both locally recurrent and metastatic castration resistant tumors. Testosterone levels in locally recurrent tumors from castrate patients were equivalent to those of BPH patients, and DHT levels were only reduced 80%, to about 0.4 ng/g (12). Compared to primary prostate tumors from untreated patients (T 0.25 ng/g, DHT 2.75 ng/g) androgen levels in metastatic CRPC tumors obtained via rapid autopsy showed 3-fold higher T levels and an inverted ratio of T to DHT (T 0.74 ng/g; DHT 0.25 ng/g) (13). Adrenal androgens have also been detected at significant levels in prostate tissue of castrate men. Prostatic levels of dihydroepiandrosterone (DHEA), DHEA-sulfate (DHEA-S), and androstenedione (AED) were decreasedby about 50% in castrate patients and far exceeded values of T and DHT in recurrent tumors (12). No decrease in prostatic levels of 5-androstenediol were found after castration (14), which is of particular significance as this androgen has been shown to bind wild type AR without being inhibited by flutamide or bicalutamide (15).
Two recently reported studies demonstrate that the addition of androgen synthesis inhibitors to castration therapy can lower prostate androgens below that achieved with standard androgen blockade. The addition of dutasteride and ketoconazole to combined androgen blockade (CAB) for 3 months prior to prostatectomy lowered prostate DHT from 0.92 ng/g (in the CAB arm) to 0.03 ng/g (16). In a second study, the potent CYP17A inhibitor abiraterone was added to LHRH agonist therapy for 3 or 6 months prior to prostatectomy. Abiraterone decreased prostate tissue DHT from 1.3 ng/g (in men treated with LHRH agonist therapy alone) to 0.18 ng/g and also decreased prostate levels of AED and DHEA (17).
Significance of intratumoral androgens in progression of CRPC
These findings clearly demonstrate that achieving castrate levels of circulating T does not eliminate androgens from the prostate tumor microenvironment. The ability of DHT in the range observed in castrate tumors (~1 nm, 0.5 to 1.0 ng/g) to activate the AR, stimulate expression of AR-regulated genes, and promote androgen mediated tumor growth has been convincingly demonstrated in both in vitro and in vivo studies (12,18-21), and is evidenced by the nearly universal rise in serum PSA that accompanies CRPC progression.
Residual tissue androgens are implicated in driving the majority of mechanisms whereby persistent AR-mediated signaling drives castration resistant disease. These mechanisms include AR overexpression, AR mutations that broaden ligand specificity and/or confer sensitivity to adrenal androgens, alterations in AR coactivators and/or corepressors that modulate AR stability and ligand sensitivity, and activation of the AR or downstream regulatory molecules by “cross talk” with other signaling pathways. Restoration of AR expression and signaling in a xenograft model was both necessary and sufficient to drive progression from androgen-dependent to castration resistant growth, allowing tumor cell proliferation in 80% lower androgen concentrations (22). Importantly, ligand binding was required for hormone refractory growth, and modest increases in AR expression were sufficient to support signaling in a low androgen environment.
The clinical relevance of intratumoral androgens in promoting CRPC tumor growth is confirmed by the clinical responses to agents targeting residual androgen pathway activity. These include historical responses described in response to adrenalectomy and/or hypophysectomy (23,24); the limited but consistent ~5% overall survival benefit seen in meta-analyses of CAB (25-27); the observation that nearly 30% of recurrent prostate tumors demonstrate at least transient clinical responses to secondary or tertiary hormonal manipulation (28); and most recently, the striking clinical response observed with novel ligand synthesis inhibitors such as abiraterone, and potent AR inhibitors such as enzalutamide (29,30). Perhaps most importantly, emerging studies suggest that response and resistance to abiraterone is associated with tumoral evidence of upregulated androgen synthesis, clearly demonstrating the importance of intratumoral androgen metabolism in CRPC tumor survival (31-33).
Pathways of androgen metabolism
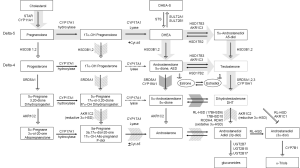
The source of residual androgens within prostate tumors of castrate men has not been fully elucidated, but is generally attributed to the uptake and conversion of circulating adrenal androgens (14,34), and somewhat more controversially, to de novo biosynthesis of androgens from progesterone or cholesterol precursors (35). Here we review the classical pathways of de novo androgen synthesis in adrenal and peripheral tissues [Figure 1, reviewed in (36)], the enzymatic pathways mediating prostate androgen metabolism, and the so called ‘back-door’ pathway of androgen synthesis. A general outline of the classical and non-classical steroidogenic pathways is provided in Figure 2.
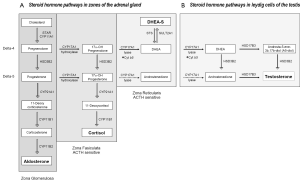
Androgen synthesis in the adrenal gland and peripheral tissues
Steroid hormone synthesis begins with transfer of a 27-carbon (C-27) cholesterol molecule from the outer mitochondrial membrane to the inner membrane by steroidogenic acute regulatory protein (STAR), followed by its conversion to the C-19 steroid, pregnenolone via CYP11A (side change cleavage enzyme). Subsequent metabolism to progesterone, mineralocorticoids, glucocorticoids (all C-21steroids), androgens (C-19) or estrogens (C-18) is dictated in a tissue-specific manner, driven by the expression of specific enzymes and catalytic cofactors.
CYP17A, expressed in the adrenal gland, testis and ovary, is a single enzyme with one active site which catalyzes sequential but independent hydroxylase and lyase reactions, both of which are required for converting C21 progestins to androgens and estrogens, either along the delta-5 pathway from pregnenolone or the delta-4 pathway from progesterone. The hydroxylase activity of CYP17A for pregnenolone and progesterone is similar, but its lyase activity for delta-5 and -4 substrates requires the activity of the cytochrome b5 cofactor, and is approximately 50 times more efficient for converting the delta-5 substrate 17-OH pregnenolone to DHEA than the delta-4 substrate 17-OH progesterone to AED (36). HSD3B enzymes catalyze the conversion of delta-5 to -4 steroids. Whereas HSD3B2 is the primary isoform expressed in adrenal, testis and ovary, HSD3B1 (10 fold more efficient) is the isoform expressed in peripheral tissues such as skin, breast, prostate, placenta and brain (36).
In adrenal steroidogenesis [Figure 1A, reviewed in (37)], the zona glomerulosa lacks CYP17A activity and produces aldosterone via the sequential activity of HSD3B2, CYP21A1, and CYP11B on pregnenolone. Both the zona fasiculata and zona reticularis express CYP17A, but the zona fasiculata does not express the cytochrome b5 cofactor required to catalyze the lyase activity of CYP17A, channeling precursors to production of glucocorticoids. The differential expression of cytochrome b5 in the zona reticularis catalyzes the lyase activity of CYP17 10-fold, leading to robust production of DHEA, followed by conversion to DHEA-S via the sulfotransferase activity of SULT2A1. The zona reticularis is characterized by low expression of HSD3B2, favoring conversion of pregnenolone to DHEA and DHEA-S, although small amounts are converted to AED (38).
Less recognized is that the human zona reticularis also expresses AKR1C3, which mediates the final step in T synthesis from AED. Notably, in a small study of 8 women, adrenal vein T levels increased 6-fold (18.5 to 116 ng/dL) before and after ACTH stimulation (39). In a much earlier study, selective adrenal vein catheterization in men also demonstrated adrenal to peripheral venous T gradients, although a compensatory increase in adrenal production of T was not observed in castrate vs. intact men (40).
Leydig cells of the testis (Figure 1B) express similar metabolic machineries, including STAR, CYP11A, and the preference of CYP17A for delta-4 substrates, allowing them to produce DHEA from cholesterol, but with several key differences, including absence of SULT2A1, preventing conversion to DHEA-S, and abundant expression of HSD3B2, which mediates the delta-4 to -5 conversion required to generate T. The final steps in T biosynthesis are catalyzed by HSD17B3 and/or HSD17B5 (called AKR1C3). HSD17B3 is primarily expressed in testicular Leydig cells, while AKR1C3 mediates production of T and DHT in peripheral tissues. The activity of HSD3B2 sand HSD17B3 thus drives the stepwise conversion of DHEA to T, via either AED or androst-5-ene-3, 17-diol (5-androstenediol or A5-diol).
Androgen synthesis in the prostate and pre-receptor control of DHT metabolism
The uptake of circulating androgens and the local synthesis of active steroids in peripheral target tissues such as breast, prostate and skin has been termed intracrinology and involves the paracrine diffusion and conversion of steroid substrates among neighboring cell types with different enzyme capacities (41). In the prostate, circulating T from the Leydig cells is converted to DHT by SRD5A2 present in both basal and luminal epithelial cells. Circulating DHEA-S can be converted to AED, T and DHT via the activity of HSD3B1, AKR1C3 and SRD5A2 present in basal epithelial cells (42,43). Circulating T or that produced in the basal cells diffuses to the AR positive luminal cells where it is then converted to DHT by SRD5A2 (41).
Prostate tissue also demonstrates epithelial cell expression of phase I (reducing) and phase II (conjugating) DHT catabolizing enzymes that act in concert to regulate access of DHT to the AR. AKR1C1 is the primary enzyme responsible for the irreversible reduction of DHT to the weak metabolite, 5α-androstane-3,17-diol (3α-androstanediol or 3α-diol, a low affinity AR ligand), whereas AKR1C2 catalyzes the reversible conversion of DHT to 5α-androstane-3,17-diol (3β-diol, a pro-apoptotic ligand of estrogen receptor beta, ER) (44). The reductase activity of AKR1C2, coupled with the reverse oxidative activity of specific 3α-HSD enzymes is a critical molecular switch regulating access of DHT to the AR (44-47).
Candidate enzymes mediating the reversible conversion of 3β-diol to DHT include RL-HSD (17BHSD6), 17BHSD10, RODH4, RDH5, and DHRS9. Transcripts of RL-HSD and 17BHSD10 are highly expressed in the prostate, however several studies suggest RL-HSD is more active in converting 3β-diol to DHT in prostate cells (48,49). Basal epithelial cell expression of RL-HSD is present at the protein level, while transcript profiling of cultured epithelial and stromal cells detects stromal expression as well (48,50). RL-HSD also acts as an epimerase to convert 3β-diol to 3α-diol, although at much higher substrate concentrations (51). Recently, RL-HSD was also shown to directly catalyze conversion of physiologic levels of DHT to 3α-diol, suggesting RL-HSD is involved in maintaining the intraprostatic balance of DHT, 3α-diol and 3β-diol (50).
The glucuronidating enzymes UGT2B15 and UTG2B17 located in prostate luminal and basal epithelial cells, respectively, irreversibly terminate the androgen signal by glucuronidation of 3β-diol (as well as T, DHT and other metabolites), and are major determinants of the androgen signal in PCa cell lines (52-54). UGDH is required to generate the substrate for glucuronide conjugation (UDP-glucuronate), and over-expression of UGDH increases the generation of glucuronidated androgens (55). Thus, the relative activity of AKR1C2 in converting DHT to 3β-diol, and of RL-HSD and UGT2B17 in competing for conversion of 3β-diol back to DHT or to 3β-diol-G, respectively, will collectively determine the amount of active steroid available for AR ligand occupancy.
Classical and backdoor pathways of androgen metabolism
In the classical pathway of androgen synthesis discussed above (Figure 2, light gray arrows), C21 steroids generated from cholesterol such as pregnenolone and progesterone are first converted to the C19 steroids DHEA and AED via sequential hydroxylase and lyase activity of CYP17A and are then acted on by HSD17B3 to generate T, with peripheral conversion of T to DHT carried out by SRD5A2 in target tissues. However, in steroidogenic tissues in which both CYP17A and SRD5A are co-expressed, an alternate route to DHT, called the ‘back-door’ pathway (Figure 2, hatched arrows) is possible wherein C21 steroids undergo 5α-reduction by SRD5A prior to being acted upon by the lyase activity of CYP17A (56). In fact, 17α-OH progesterone is a better substrate for SRD5A (especially SRD5A1) than either AED or T (57). Since 17α-OH dihydroprogesterone (the 5α-reduced product of 17α-OH progesterone) is a poor substrate for the lyase activity of CYP17A, synthesis proceeds via the 3α-reduction of 17α-OH dihydroprogesterone by ARK1C2, which yields 17α-OH allo-pregnanolone, an excellent substrate for CYP17A lyase activity that is minimally dependent on cytochrome b5 (58). Androsterone generated by the lyase activity of CYP17A is then acted upon by HSD17B3 or AKR1C3 to generate 3β-diol. In this case, a reverse oxidative step catalyzed by RL-HSD (not required in the classical pathway) is required to generate DHT (36). This pathway, e.g., 5α-reduction of C21 steroids prior to the action of CYP17A lyase, occurs in the testis of the immature mouse and the tammar wallaby, is also hypothesized to occur in ovarian hyperandrogenism and polycystic ovarian syndromes, as the human ovary expresses both CYP17A and SRD5A (56).
Interestingly, production of DHT in mouse testis via this mechanism is specifically mediated by type 1 and not the type 2 isoform of SRD5A (59). This observation is of relevance to prostatic androgen metabolism in that a clear shift from SRD5A2 to SRD5A1 expression occurs in the transition from benign to neoplastic prostate tissue (discussed below). Moreover, human CYP17A displays markedly more robust lyase activity for the 5α-reduced progesterone intermediate 17α-OH allo-pregnanolone than for the classical substrates 17α-OH pregnenolone or 17α-OH progesterone, such that the combination of increased SRD5A1 activity in conjunction with expression of CYP17A in PCa tissue may favor de novo synthesis via the backdoor pathway over the classical pathway (60). Importantly, while these ‘backdoor’ pathways to DHT bypass conventional intermediates of AED and T, it is worth emphasizing that the backdoor pathway requires the same enzymatic conversions which produce DHT via the conventional pathway; all that differs is the order in which the enzymes mediate the reactions.
Altered expression of steroidogenic enzymes in progression to CRPC
Primary PCa and castration resistant tumors are characterized by a number of changes in steroidogenic gene expression that are consistent with either promoting conversion of adrenal androgens to DHT, inhibiting conversion of DHT to inactive metabolites, or in case of CRPC tumors, mediating de novo synthesis of androgens from cholesterol and/or progestin precursors. Here we review the alterations observed in prostate tumors during the progression to CRPC and discuss implications of these changes for determining intra-tumor androgen levels.
Altered expression of steroidogenic genes in primary PCa
Perhaps the most consistently observed alteration in prostate tumors is a subtotal loss of tumoral SRD5A2, the principle isoform of this enzyme expressed in benign prostate tissue (61), and a relative shift in primary and recurrent prostate tumors to expression of SRD5A1 (34,62,63) [although some studies have shown Gleason grade-related increases in both SRD5A1 and SRD5A2 (64)]. The significance of this shift was recently elucidated by the group of Sharifi who demonstrated that (I) the 5α-reduction of AED to 5α-androstanedione is a required step for DHT synthesis in PCa cells (rather than direct 5α-reduction of T to DHT); (II) this conversion is specifically mediated by SRD5A1; and (III) that in PCa cells T and AED are actually negligible substrates for SRD5A2 (60) (possibly related to the altered redox environment of tumor cells as SRD5A1 and 2 have different pH optima). These data support previous findings that SRD5A activity in PCa cells has a preference for AED rather than T (65), as well as initial studies of SRD5A1 which found AED to be a better substrate for 5α-reduction than T (66,67).
Sharifi et al. call this the 5α-androstandione pathway (Figure 2, dark arrow) and suggest that the upregulation of SRD5A1 observed in the transition to CRPC reflects selection of tumors cells capable of efficiently synthesizing DHT via this pathway. Interestingly, a recent report demonstrated that progression to CRPC was correlated with a higher pre-treatment ratio of T to DHT in prostate biopsies taken before the start of ADT [T:DHT ratio 0.19 pg/mg (0.98 to 4.92 pg/mg) vs. 0.05 pg/mg (0.45 to 16.89 pg/mg) in patients who did not develop CRPC] (68). It is tempting to speculate that this elevated ratio of T to DHT may reflect tumor cells with pre-treatment loss of SRD5A2 activity, followed by induction of SRD5A1-mediated DHT production via 5α-androstanedione under the selection pressure of ADT. Altered expression of a third SRD5A isozyme, SRD5A3, has also been reported, with increased expression observed in primary and castration recurrent prostate tumors (69). The importance and/or activity of this enzyme in PCa progression awaits further evaluation (70).
Differential changes in the expression of reductive and oxidative enzyme pairs favoring the conversion of inactive diones to active androgens (e.g., AED to T, or androstanedione to DHT) have been observed in primary prostate tumors, including increased tumor expression of the reductive enzymes HSD17B3 (71) and AKR1C3 (34,43,72), and decreased expression of the oxidative enzyme catalyzing the reverse reaction, HSD17B2 (71,73), suggesting a shift in tumoral androgen metabolism to the formation of T and DHT. While increased prostate tumor expression of HSD17B4, which has unidirectional oxidative activity, has been observed, this isoform (also known as D-bifunctional protein or DBP) has a unique peroxisomal targeting sequence and acts primarily in peroxisomal -chain oxidation of fatty acids (74).
Similarly, primary PCa demonstrates a selective loss of both AKR1C2 and AKR1C1 versus paired benign tissues, accompanied by a reduced capacity to metabolize DHT to 3β-diol, resulting in increased tumoral DHT levels (47). Increased expression of HSD17B10, one of the oxidative enzyme capable of mediating the back conversion of 3β-diol to DHT, has also been observed in malignant epithelial cells compared to normal, similarly consistent with an increased capacity to generate DHT in tumor tissue (75). In contrast, epithelial expression of RL-HSD (which can mediate either conversion of 3β-diol to DHT or of DHT to 3α-diol) is lost in primary PCa, which is hypothesized to reflect loss of the 3β-diol/ER mediated growth inhibition pathway during malignant transformation (50).
Another enzyme which may modulate prostate tissue androgen levels is SULT2B1, which shows selective loss of expression in tumor vs. benign prostate epithelial cells (76). While SULT2A1 is the primary phase II enzyme responsible for sulfonation in the adrenal gland, SULT2B1b is highly expressed in the prostate and may limit the pool of unconjugated DHEA available for conversion to AED. This is consistent with a report demonstrating increased DHEA-stimulated LNCaP proliferation in cells with knockdown of SULT2B1b (77).
Altered expression of steroidogenic genes in castration resistant prostate tumors
CRPC tumors demonstrate altered expression of numerous genes in the steroid synthesis pathway, including genes involved in cholesterol metabolism, de novo steroidogenesis, as well as utilization of adrenal androgens, suggesting that castration resistant tumors may have the ability to utilize cholesterol, progesterone and/or adrenal precursors for conversion to T and DHT (13,34). Changes related to cholesterol metabolism include increased expression of squalene epoxidase (SQLE), the rate-limiting enzyme in cholesterol synthesis, as well other genes in this pathway such as HMG-CoA synthase, squalene synthetase and lanosterol synthase (35). In a study comparing CRPC with primary tumors, the relative expression of numerous transcripts involved in de novo androgen biosynthesis and adrenal androgen utilization were altered, including increased expression of HSD3B2 (1.8), AKR1C3 (5.3), SRD5A1 (2.1), SRD5A2 (0.54), AKR1C2 (3.4), AKR1C1 (3.1) and UGT2B15 (3.5). Another study of CRPC metastases in which elevated levels of tumor T and DHT were also measured (T 0.74 ng/g, DHT 0.25 ng/g), showed elevated expression of STAR, CYP17A, HSD3B1/2, HSD17B3, AKR1C3, SRD5A, UGT2B15/17, CYP19A and decreased SRD5A2 (13,78,79). Interestingly, CYP17A has also been demonstrated to have squalene epoxidase activity in assays using recombinant CYP17A and in a mouse Leydig tumor cell line (80), suggesting it may have a dual role in CRPC steroid metabolism. Other studies have not specifically found increased expression of CYP17A in CRPC tumors, but have demonstrated similar findings suggestive of intracrine utilization of adrenal androgens, including increased expression of HSD17B3 and AKR1C3 (34,81,82). Also of note, AKR1C3 has recently been identified as an AR coactivators and thus may play dual roles in promoting ligand synthesis and AR activation (83).
A gain of function SNP in HSD3B1 (1245C; N367T, population frequency 22%) has recently been identified as a somatic mutation in CRPC tumors (84). Three of 25 CRPC tumors with wild type germline DNA at this site had acquired the gain of function mutation in the tumor, and 3 of 11 CRPC tumors with heterozygous germline DNA, showed loss of heterozygosity of the wild type allele. Expression of this variant resulted in increased protein levels of HSD3B1, rendered the protein resistant to ubiquitination and degradation, and lead to increased levels of intratumoral DHT production. Compared to the poor conversion of DHEA to AED in LAPC4 cells which do not have this mutation, the mutant allele was shown to account for the efficient flux of DHEA to AED in LNCaP cells, and was also detected in the VCaP cell line.
Notably, the expression of enzymes involved in de novo steroidogenesis, including MLN64 (homolog of STAR), CYP11A, CYP17A and HSD3B has also been demonstrated in primary prostate tissues (78,85). While a role for de novo steroidogenesis per se in primary prostate tumors is less likely, these observations suggest that the selection pressure of ADT may lead to upregulated expression of these enzymes and reconstitution of tumor androgen levels in CRPC.
Intracrine steroidogenesis in the continuum from normal prostate to CRPC
The ability of prostate tissue and prostate tumors to mediate the intracrine conversion of adrenally derived androgens or cholesterol to downstream androgens of T and DHT has been evaluated in normal rat and human prostate, in primary prostate tumors, in CRPC tumors, and in vitro and in vivo models of CRPC. Here we review the evidence in each of these setting that demonstrate the activity of steroidogenic pathways in the continuum from normal prostate to CRPC.
Evaluation of steroidogenesis in normal prostate and PCa tissue
A number of early studies attempted to directly examine the steroidogenic ability of rat and human prostate tissue by evaluating the conversion of exogenously administered radiolabeled-adrenal androgens to T or DHT. Bruchovsky administered radioactively labeled androgens including T, DHT, and the adrenal androgens DHEA and AED to castrated male rats and evaluated prostatic metabolites at 60 minutes after injection (86). Following administration of DHEA approximately 1% and 8% of the recovered radioactivity was found in T and DHT respectively, with AED it was 2% and 12%, respectively, compared to 37% conversion of exogenously administered T into DHT. Labrie et al. demonstrated that administering DHEA or AED to castrate adult rats at levels found in the serum of adult men led to increased prostatic DHT levels and increases in ventral prostate weight (87). In the Dunning R3327 prostate carcinoma model, administration of adrenal androgens to castrate male rats increased tumor DHT levels and stimulated tumor growth to the level of intact controls (88).
In studies of human prostate tissue, Harper et al. evaluated prostate androgen metabolism by infusing eugonadal men with 3H-T, 3H-AED or 3H-DHEA-sulfate (DHEA-S) thirty minutes prior to performing radical prostatectomy for BPH (89). The major metabolite present in prostate tissue after 3H-T infusion was DHT (about 65% conversion). Infusion of 3H-AED resulted in approximately 7-10% radioactivity associated with either T or DHT. 3H-DHEA-S was primarily converted to DHEA (70-90%), with 1-3% conversion to T, DHT and AED. Consistent with these observations, a more recent study using mass spectrometry to identify metabolites formed from ex-vivo incubation of human prostate homogenate with DHEA demonstrated production of 5-androstenediol, T, DHT and androsterone (90). Together, these studies in rat and human prostate tissues suggest that while the most efficient substrate for DHT production in non-tumor prostate tissue is T, a limited amount of DHT is also formed from exogenous DHEA or AED, consistent with intracrine steroidogenesis.
In PCa tissues Acevedo et al. investigated the metabolism of C14 progesterone in primary cancer, but did not observe significant metabolic conversion beyond the formation of immediate progesterone derivatives (91). This finding is not necessarily unexpected, as studies have now clearly demonstrated that it is CRPC tumors in which steroidogenic genes capable of de novo synthesis are upregulated. Klein et al. evaluated the presence of adrenal androgens and steroid metabolizing activity (including SRD5A, HSD3B, and HSD17B) ex vivo in hormone naive tumors and lymph node metastases. Although malignant tissue had a sub-total loss of SRD5A activity, they found that primary tumors and metastases possessed the capacity to metabolize adrenal androgen precursors along the pathway to DHT (61). Di Silverio et al. demonstrated the conversion of DHEA-S to DHEA within PCa tissue extracts from both eugonadal and castrate men (92), and Klein et al. subsequently confirmed the presence of the steroid sulfatase required for conversion of DHEA-S to DHEA within prostate epithelial tissue (93). Consistent with their observation that the primary route to DHT in PCa cells is from AED to androstenedione rather than from AED to T, Sharifi’s group demonstrated robust conversion of AED to androstenedione and limited to no metabolism of AED to T in biopsy tissue from two patients with CRPC (60).
Experimental models of de novo steroidogenesis in CRPC
Studies using in vitro and in vivo models of CRPC support the concept of intratumoral androgen synthesis, including both adrenal androgen utilization and de novo androgen synthesis (94). Numerous studies using CRPC xenograft models in castrate mice have demonstrated measurement of substantial intratumor androgen levels (31,32,95-99). As rodent adrenal glands do not synthesize significant amounts of adrenal androgens, these findings are suggestive of de novo steroidogenesis from cholesterol or progesterone precursors. Notably, circulating levels of exogenously administered cholesterol were associated with tumor size (R=0.3957, P=0.0049) and intratumoral T levels (R=0.41, P=0.0023) in subcutaneous LNCaP tumors grown in hormonally intact mice, and were directly correlated with tumoral expression of CYP17A (R=0.4073, P=0.025). Since the hypercholesterolemia did not raise circulating androgen levels, these data suggest the administered cholesterol led to increased intratumoral androgens via de novo steroidogenesis.
More directly addressing this question, a number of groups have carried out in vitro studies with radiolabeled cholesterol precursors to demonstrate intratumoral conversion to downstream metabolites. The androgen-independent LNCaP derivative (C81) showed higher expression of STAR, CYP11A and CYP17A compared to its androgen-dependent counterpart (C33) and was shown to directly convert radioactive cholesterol into T (100). Increases in expression of genes responsible for accumulation of free cholesterol and cholesterol synthesis including LDLR, SRB1, ABCA1, STAR, ACAT, HMG-CoA and CYP11A were demonstrated in a xenograft LNCaP model (97,101,102) (as well as increases in transcripts encoding CYP17A, AKR1C1, AKR1C2, AKR1C3, HSD17B2, and SRD5A1) (101). Conversion of C14-acetic acid to DHT was observed in these xenografts, and tumors were shown to metabolize H3-progesterone to six different intermediates upstream of 5α-DHT, suggesting occurrence of steroidogenesis via both classic and “backdoor” pathways (101). In a study of six prostate cell lines (LnCaP, 22Rv1, DU145, RWPE1, PC3 and ALVA4), expression of CYP11A, CYP17A, HSD3B2, 17BSHD3 was detected in all, with conversion of C14-labled cholesterol to T and DHT demonstrated in each cell line, albeit with different efficiencies (78). It should be noted that other studies have not detected expression of CYP17A or evidence for de novo steroidogenesis in PCa cell lines (103,104).
Exogenous influences on intratumoral androgen biosynthesis
A number of exogenous factors including cytokines, growth factors and paracrine cellular interactions have been found to promote steroid production in PCa cell lines. IL-6 is implicated in cross-talk and regulation of AR activity and PCa growth, but may also play a role in modulating androgen synthesis. Treatment of LNCaP cells with IL-6 induced the expression of steroidogenic enzymes including CYP11A, HSD3B2, AKR1C3 and HSD17B3, and increased levels of T in lysates of cells grown in serum free media by 2 fold (105).
In a study designed to evaluate the effects of insulin on steroidogenesis, exposure of LNCaP cells to insulin caused an increase in transcript levels of cholesterol and steroid synthesizing genes, including SREBP1, STAR, CYP11A, CYP17A, HSD3B2, HSD17B3, and SRD5A1, which were confirmed at the protein level for a number of genes including CYP11A1 and CYP17A1. In parallel, insulin increased intracellular levels of pregnenolone, 17α-OH progesterone, DHEA and T, and incubation of insulin-treated LNCaP and VCaP cells with C14-acetate resulted in detection of radiolabeled pregnan-3,20-dione, AED, T and androsterone (99). In similar studies evaluating the effect of IGF2 on steroidogenesis, these authors demonstrated increased conversion of C14-acetate to pregnan-3,20-dione, pregnan-3,17-diol-20-one, androsterone, AED, and T (106).
Receptors for luteinizing hormone (LH), the target of LH releasing hormone (LHRH) agonist therapy in the brain, have also been demonstrated in PCa specimens and may play a role in steroidogenesis (107). Exposure of both androgen-sensitive (LNCaP) and androgen-independent (22RV1 and C4-2B) PCa cell lines to LH increased the protein expression of steroidogenic enzymes including STAR, CYB5B, CYP11A, and 3BHSD, and a 2.5 fold increase in progesterone synthesis was observed in LH treated C4-2B cells compared to controls (108). These data suggest that LH may have a role in the regulation of steroid biosynthesis in PCa cells and identify the LH receptor as a potential therapeutic target.
Several studies have indicated that bone-marrow and PCa-derived stromal cells may play an important role in facilitating androgen biosynthesis in PCa cells. Whereas DHEA induced little or no PSA expression in monocultures of LAPC-4 PCa cells, co-culture with PCa-associated stromal cells resulted in marked stimulation of PSA expression, likely mediated by stromal cell generation of T from DHEA (as T was detected in a time and dose-dependent manner in PCa-stromal cell monocultures treated with DHEA) (109). Similarly, the impact of DHEA on PSA promoter activity in LNCaP cells was markedly enhanced in the presence of PCa-derived stromal cells (110). Knockdown of AR in the LNCaP cells abrogated this effect, while coculture with PCa-stromal cells transfected with AR shRNA did not, suggesting paracrine factors secreted by the stromal cells act on the LNCaP AR. Furthermore, following DHEA treatment, T and DHT concentrations were ~5-fold higher in the PCa-stromal/LNCaP coculture vs. the LNCaP monoculture. Interestingly, normal-prostate stroma, bone-marrow stroma, lung stroma and bone-derived stromal cells also induced an increase in PSA expression, although the strongest effects were noted with PCa-stromal cells. In a separate study of bone-marrow stromal cells, resting mesenchymal cells were found to express HSD3B and SRD5A protein, while incubation with DHEA resulted in the additional expression of HSD17B5 (111). These findings indicate that metabolism of androgen precursors in PCa-associated stromal cells may facilitate and/or potentiate the maintenance of intratumoral androgen levels in CRPC tumors.
Together these studies provide evidence supporting the role of steroidogenesis in reactivating AR signaling in CRPC, and highlight the interplay between cytokines, growth factors, and paracrine stromal and epithelial cell interactions in this mechanism.
Response and resistance to potent steroidogenesis inhibition in CRPC
Collectively, these studies demonstrate the capacity of primary and castration resistant prostate tumors to carry out the intracrine conversion of adrenal androgens to DHT, while the in vitro and in vivo experimental models clearly show that PCa cells are capable of de novo steroidogenesis starting from cholesterol and/or progesterone precursors. These findings cannot address the efficiency with which these pathways are active in human CRPC tumors in situ, but they strongly support the premise that the residual androgens measured in CRPC tumors reflect the increased expression and activity of enzymes mediating de novo steroidogenesis and adrenal androgen utilization. These data provide mechanistic support for the role of intracrine androgen production in maintaining the tumor androgen microenvironment in CRPC and underscore these metabolic pathways as critical therapeutic targets.
Given its central role in the production of either adrenal or tumor-derived androgens, CYP17A has emerged as a primary target of novel therapeutics. Abiraterone, a pregnenolone derivative that acts as a selective irreversible inhibitor of both the hydroxylase and lyase activity CYP17A, is the first of these agents to enter clinical practice. While clinical responses have been impressive, not all patients respond, the duration of response is variable, and a majority of men eventually progress with a rising PSA. Although the mechanisms determining response and mediating resistance to CYP17A inhibition have not been fully elucidated, emerging clinical and pre-clinical data suggest several possibilities.
Perhaps most importantly, pre-clinical studies provided the first in vivo confirmation that the clinical effect of abiraterone was associated with suppression of tumor androgen levels. Clinical studies have clearly demonstrated abiraterone-mediated suppression of serum androgens, including suppression of DHEA by approximately 75% and of DHEA-S, AED, and T to essentially undetectable (112,113). As well, higher levels of AR and CYP17A staining in pre-treatment tumor-infiltrated bone marrow biopsies from men with CRPC were associated with longer responses to abiraterone treatment, supporting CYP17A mediated androgen production as the target of abiraterone activity (41). However, the efficacy of abiraterone in suppressing tumor androgens in men with CRPC remains to be demonstrated.
In this regard, treatment of castration resistant LuCaP35 and LuCaP23 xenografts significantly inhibited tumor growth, serum PSA, and intratumoral androgen levels, supporting the hypothesis that abiraterone’s primary mechanism of action is through effects on tissue androgens (31). Seven days after starting treatment levels of T and DHT decreased from 0.49 to 0.03 pg/mg and 2.65 to 0.23 pg/mg, respectively in LuCaP23, and from 0.69 to 0.02 pg/mg and 3.5 to 0.24 pg/mg in LuCaP35. A similar impact of abiraterone on T and DHT levels was observed in separate study of castration resistant VCaP tumors (32). Notably, while androgen levels remained suppressed in LuCaP23 tumors recurring after therapy, increasing levels of T and DHT were observed in LuCaP35 tumors recurring on abiraterone.
Further evaluation demonstrated that these CRPC models responded to CYP17A inhibition with multiple mechanisms directed at maintaining AR signaling. This included upregulated expression of full length AR and ligand independent AR variants, as well as induction of steroidogenic genes (including the target gene, CYP17A), several of which showed strong correlations with DHT levels in recurrent tumors. Moreover, tumor biopsies from patients treated with the CYP17A inhibitor ketoconazole also demonstrated increased expression of transcripts encoding CYP17A compared to biopsies from CRPC patients not treated with ketoconazole (114).
These findings are consistent with clinical observations that patients progressing on abiraterone have a rise in PSA, suggesting reactivation of AR signaling. Development of resistance to abiraterone has not been associated with a rise in serum androgen levels or in bone marrow aspirate T levels (although 5α-androstanedione may be more appropriate to assess if the route to DHT bypasses T). However, numerous studies (reviewed above) show that circulating androgen levels do not reflect tumor cell androgen concentrations. Thus, in the setting of tumor progression on abiraterone, the rationale for focusing further therapeutic efforts on more potent AR antagonists and agents suppressing AR ligands remains strong.
Conclusions
Data regarding the molecular response of PCa to hormone therapy continues to emerge, providing critical insight into cellular growth and signaling pathways that may be exploited as therapeutic targets. The presence of residual androgens and persistent activation of the AR signaling axis in CRPC suggest that a multi-targeted treatment approach to ablate all contributions to AR signaling within the prostate tumor will be required for optimal anti-tumor efficacy.
The molecular alterations occurring in CRPC tumors following abiraterone treatment suggest tumor-specific methods of addressing resistance, either through optimizing steroidogenic blockade or by inhibiting AR signaling. Importantly, a 2 to 3 fold increase in AR expression can render low androgen levels (in the range detected in the abiraterone-treated tumors) physiologically relevant in promoting AR driven growth (22). Combining CYP17A blockade with inhibitors of other critical components of the pathway such as HSD3B1 or SRD5A2 or with AR inhibitors could offset adaptive upregulation of CYP17A (115). Abiraterone at higher (but clinically achievable) concentrations can strongly inhibit HSD3B1 and 2 (116), and can antagonize the promiscuous T877A mutant AR (117), providing a rationale for dose-escalation of abiraterone at time of progression. However, data demonstrating the induction of full length and ligand-independent AR splice variants in abiraterone-treated tumors suggests combined strategies directed at targeting ligand synthesis with AR inhibitors may have the greatest efficacy.
The introduction of potent steroidogenic inhibitors such as abiraterone and novel AR inhibitors such as MDV3100 holds significant promise for improving the treatment of men with CRPC. However, the optimal timing, sequence, and potential combinatorial strategies using new AR pathway inhibitors are critical unanswered questions. Delineating mechanisms and biomarkers of resistance will be critical for rational trial design and for the stratification of men to treatment strategies with the highest likelihood of durable efficacy.
Acknowledgements
Support: Pacific Northwest Prostate Cancer SPORE P50 CA97186; Department of Defense CDMRP; Prostate Cancer Foundation; Damon Runyon Cancer Research Foundation (Damon Runyon-Genentech Clinical Investigator Award CI-40-08).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Geller J, Albert J, Loza D, et al. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab 1978;46:440-4. [PubMed]
- Geller J, Albert J, Yen SS, et al. Medical castration of males with megestrol acetate and small doses of diethylstilbestrol. J Clin Endocrinol Metab 1981;52:576-80. [PubMed]
- Liu J, Geller J, Albert J, et al. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metab 1985;61:129-33. [PubMed]
- Liu J, Albert J, Geller J. Effects of androgen blockade with ketoconazole and megestrol acetate on human prostatic protein patterns. Prostate 1986;9:199-205. [PubMed]
- Geller J, Albert J. Effects of castration compared with total androgen blockade on tissue dihydrotestosterone (DHT) concentration in benign prostatic hyperplasia (BPH). Urol Res 1987;15:151-3. [PubMed]
- Geller J, Liu J, Albert J, et al. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin Endocrinol (Oxf) 1987;26:155-61. [PubMed]
- Labrie F, Dupont A, Belanger A, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med 1982;5:267-75. [PubMed]
- Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab 2006;91:3850-6. [PubMed]
- Forti G, Salerno R, Moneti G, et al. Three-month treatment with a long-acting gonadotropin-releasing hormone agonist of patients with benign prostatic hyperplasia: effects on tissue androgen concentration, 5 alpha-reductase activity and androgen receptor content. J Clin Endocrinol Metab 1989;68:461-8. [PubMed]
- Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res 2004;10:7121-6. [PubMed]
- Nishiyama T, Ikarashi T, Hashimoto Y, et al. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J Urol 2007;178:1282-8. [PubMed]
- Mohler JL, Gregory CW, Ford OH 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004;10:440-8. [PubMed]
- Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447-54. [PubMed]
- Mizokami A, Koh E, Fujita H, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res 2004;64:765-71. [PubMed]
- Miyamoto H, Yeh S, Lardy H, et al. Delta5-androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. Proc Natl Acad Sci U S A 1998;95:11083-8. [PubMed]
- Mostaghel EA, Nelson P, Lange PH, et al. Neoadjuvant pathway suppression prior to prostatectomy. Proc Am Soc Clin Onc 2012.
- Taplin ME, Montgomery RB, Logothetis C, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): results of a randomized phase II study. J Clin Oncol 2012;30:abstr 4521.
- Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer 1999;81:242-51. [PubMed]
- Gregory CW, Johnson RT Jr, Mohler JL, et al. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res 2001;61:2892-8. [PubMed]
- Gregory CW, Hamil KG, Kim D, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res 1998;58:5718-24. [PubMed]
- Mohler JL, Morris TL, Ford OH 3rd, et al. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate 2002;51:247-55. [PubMed]
- Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004;10:33-9. [PubMed]
- Greenberg E. Endocrine therapy in the management of prostatic cancer. Clin Endocrinol Metab 1980;9:369-81. [PubMed]
- Robinson MR, Shearer RJ, Fergusson JD. Adrenal suppression in the treatment of carcinoma of the prostate. Br J Urol 1974;46:555-9. [PubMed]
- Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002;95:361-76. [PubMed]
- Schmitt B, Bennett C, Seidenfeld J, et al. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev 2000.CD001526. [PubMed]
- Caubet JF, Tosteson TD, Dong EW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 1997;49:71-8. [PubMed]
- Small EJ, Ryan CJ. The case for secondary hormonal therapies in the chemotherapy age. J Urol 2006;176:S66-71. [PubMed]
- de Bono JS. Abiraterone acetate improves survival in metastatic castration-resistant prostate cancer: Phase III results. 2010 European Society for Medical Oncology. Milan, 2010.
- Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437-46. [PubMed]
- Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 2011;17:5913-25. [PubMed]
- Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011;71:6503-13. [PubMed]
- Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 2012;30:637-43. [PubMed]
- Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815-25. [PubMed]
- Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol 2004;164:217-27. [PubMed]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81-151. [PubMed]
- Rainey WE, Carr BR, Sasano H, et al. Dissecting human adrenal androgen production. Trends Endocrinol Metab 2002;13:234-9. [PubMed]
- Endoh A, Kristiansen SB, Casson PR, et al. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 1996;81:3558-65. [PubMed]
- Nakamura Y, Hornsby PJ, Casson P, et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab 2009;94:2192-8. [PubMed]
- Sanford EJ, Paulson DF, Rohner TJ Jr, et al. The effects of castration on adrenal testosterone secretion in men with prostatic carcinoma. J Urol 1977;118:1019-21. [PubMed]
- Labrie F, Luu-The V, Lin SX, et al. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol 2000;25:1-16. [PubMed]
- Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 2008;22:207-21. [PubMed]
- Fung KM, Samara EN, Wong C, et al. Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer 2006;13:169-80. [PubMed]
- Penning TM, Bauman DR, Jin Y, et al. Identification of the molecular switch that regulates access of 5[alpha]-DHT to the androgen receptor. Mol Cell Endocrinol 2007;265-266:77-82. [PubMed]
- Rizner TL, Lin HK, Peehl DM, et al. Human Type 3 3{alpha}-Hydroxysteroid Dehydrogenase (Aldo-Keto Reductase 1C2) and Androgen Metabolism in Prostate Cells. Endocrinology 2003;144:2922-32. [PubMed]
- Ji Q, Chang L, VanDenBerg D, et al. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate 2003;54:275-89. [PubMed]
- Ji Q, Chang L, Stanczyk FZ, et al. Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre-receptor regulator of androgen receptor signaling. Cancer Res 2007;67:1361-9. [PubMed]
- Bauman DR, Steckelbroeck S, Williams MV, et al. Identification of the major oxidative 3{alpha}-hydroxysteroid dehydrogenase in human prostate that converts 5{alpha}-androstane-3{alpha},17{beta}-diol to 5{alpha}-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol 2006;20:444-58. [PubMed]
- Mohler JL, Titus MA, Bai S, et al. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res 2011;71:1486-96. [PubMed]
- Muthusamy S, Andersson S, Kim HJ, et al. Estrogen receptor beta and 17beta-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proc Natl Acad Sci U S A 2011;108:20090-4. [PubMed]
- Huang XF, Luu-The V. Molecular characterization of a first human 3(alpha-->beta)-hydroxysteroid epimerase. J Biol Chem 2000;275:29452-7. [PubMed]
- Guillemette C, Lévesque E, Beaulieu M, et al. Differential regulation of two uridine diphospho-glucuronosyltransferases, UGT2B15 and UGT2B17, in human prostate LNCaP cells. Endocrinology 1997;138:2998-3005. [PubMed]
- Chouinard S, Barbier O, Bélanger A. UDP-glucuronosyltransferase 2B15 (UGT2B15) and UGT2B17 enzymes are major determinants of the androgen response in prostate cancer LNCaP cells. J Biol Chem 2007;282:33466-74. [PubMed]
- Chouinard S, Pelletier G, Bélanger A, et al. Cellular specific expression of the androgen-conjugating enzymes UGT2B15 and UGT2B17 in the human prostate epithelium. Endocr Res 2004;30:717-25. [PubMed]
- Wei Q, Galbenus R, Raza A, et al. Androgen-stimulated UDP-glucose dehydrogenase expression limits prostate androgen availability without impacting hyaluronan levels. Cancer Res 2009;69:2332-9. [PubMed]
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 2004;15:432-8. [PubMed]
- Auchus RJ. Non-traditional metabolic pathways of adrenal steroids. Rev Endocr Metab Disord 2009;10:27-32. [PubMed]
- Gupta MK, Guryev OL, Auchus RJ. 5alpha-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys 2003;418:151-60. [PubMed]
- Mahendroo M, Wilson JD, Richardson JA, et al. Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways. Mol Cell Endocrinol 2004;222:113-20. [PubMed]
- Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2011;108:13728-33. [PubMed]
- Klein H, Bressel M, Kastendieck H, et al. Androgens, adrenal androgen precursors, and their metabolism in untreated primary tumors and lymph node metastases of human prostatic cancer. Am J Clin Oncol 1988;11 Suppl 2:S30-6. [PubMed]
- Luo J, Dunn TA, Ewing CM, et al. Decreased gene expression of steroid 5 alpha-reductase 2 in human prostate cancer: implications for finasteride therapy of prostate carcinoma. Prostate 2003;57:134-9. [PubMed]
- Titus MA, Gregory CW, Ford OH 3rd, et al. Steroid 5{alpha}-Reductase Isozymes I and II in Recurrent Prostate Cancer. Clin Cancer Res 2005;11:4365-71. [PubMed]
- Thomas LN, Douglas RC, Lazier CB, et al. Levels of 5[alpha]-Reductase Type 1 and Type 2 are Increased in Localized High Grade Compared to Low Grade Prostate Cancer. J Urol 2008;179:147-51. [PubMed]
- Negri-Cesi P, Motta M. Androgen metabolism in the human prostatic cancer cell line LNCaP. J Steroid Biochem Mol Biol 1994;51:89-96. [PubMed]
- Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5 alpha-reductase isozymes. J Biol Chem 1993;268:17404-12. [PubMed]
- Samson M, Labrie F, Zouboulis CC, et al. Biosynthesis of dihydrotestosterone by a pathway that does not require testosterone as an intermediate in the SZ95 sebaceous gland cell line. J Invest Dermatol 2010;130:602-4. [PubMed]
- Shibata Y, Suzuki K, Arai S, et al. Impact of pre-treatment prostate tissue androgen content on the prediction of castration-resistant prostate cancer development in patients treated with primary androgen deprivation therapy. Andrology 2013;1:505-11. [PubMed]
- Godoy A, Kawinski E, Li Y, et al. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate 2011;71:1033-46. [PubMed]
- Azzouni F, Godoy A, Li Y, et al. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol 2012;2012:530121.
- Koh E, Noda T, Kanaya J, et al. Differential expression of 17beta-hydroxysteroid dehydrogenase isozyme genes in prostate cancer and noncancer tissues. Prostate 2002;53:154-9. [PubMed]
- Lin HK, Steckelbroeck S, Fung KM, et al. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3a-hydroxysteroid dehydrogenase/type 5 17b-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids 2004;69:795-801. [PubMed]
- Elo JP, Akinola LA, Poutanen M, et al. Characterization of 17beta-hydroxysteroid dehydrogenase isoenzyme expression in benign and malignant human prostate. Int J Cancer 1996;66:37-41. [PubMed]
- Zha S, Ferdinandusse S, Hicks JL, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate 2005;63:316-23. [PubMed]
- He XY, Yang YZ, Peehl DM, et al. Oxidative 3alpha-hydroxysteroid dehydrogenase activity of human type 10 17beta-hydroxysteroid dehydrogenase. J Steroid Biochem Mol Biol 2003;87:191-8. [PubMed]
- Seo YK, Mirkheshti N, Song CS, et al. SULT2B1b sulfotransferase: induction by vitamin D receptor and reduced expression in prostate cancer. Mol Endocrinol 2013;27:925-39. [PubMed]
- He D, Falany CN. Inhibition of SULT2B1b expression alters effects of 3beta-hydroxysteroids on cell proliferation and steroid hormone receptor expression in human LNCaP prostate cancer cells. Prostate 2007;67:1318-29. [PubMed]
- Bennett NC, Hooper JD, Lambie D, et al. Evidence for steroidogenic potential in human prostate cell lines and tissues. Am J Pathol 2012;181:1078-87. [PubMed]
- Pâquet S, Fazli L, Grosse L, et al. Differential expression of the androgen-conjugating UGT2B15 and UGT2B17 enzymes in prostate tumor cells during cancer progression. J Clin Endocrinol Metab 2012;97:E428-32. [PubMed]
- Liu Y, Yao ZX, Papadopoulos V. Cytochrome P450 17alpha hydroxylase/17,20 lyase (CYP17) function in cholesterol biosynthesis: identification of squalene monooxygenase (epoxidase) activity associated with CYP17 in Leydig cells. Mol Endocrinol 2005;19:1918-31. [PubMed]
- Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res 2010;70:1256-64. [PubMed]
- Mitsiades N, Sung CC, Schultz N, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res 2012;72:6142-52. [PubMed]
- Yepuru M, Wu Z, Kulkarni A, et al. Steroidogenic Enzyme AKR1C3 is a Novel Androgen Receptor-Selective Coactivator That Promotes Prostate Cancer Growth. Clin Cancer Res 2013;19:5613-25. [PubMed]
- Chang KH, Li R, Kuri B, et al. A Gain-of-Function Mutation in DHTSynthesis in Castration Resistant Prostate Cancer. Cell 2013;154:1074-84. [PubMed]
- Stigliano A, Gandini O, Cerquetti L, et al. Increased metastatic lymph node 64 and CYP17 expression are associated with high stage prostate cancer. J Endocrinol 2007;194:55-61. [PubMed]
- Bruchovsky N. Comparison of the metabolites formed in rat prostate following the in vivo administration of seven natural androgens. Endocrinology 1971;89:1212-22. [PubMed]
- Labrie C, Simard J, Zhao HF, et al. Stimulation of androgen-dependent gene expression by the adrenal precursors dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology 1989;124:2745-54. [PubMed]
- Schiller CD, Schneider MR, Hartmann H, et al. Growth-stimulating effect of adrenal androgens on the R3327 Dunning prostatic carcinoma. Urol Res 1991;19:7-13. [PubMed]
- Harper ME, Pike A, Peeling WB, et al. Steroids of adrenal origin metabolized by human prostatic tissue both in vivo and in vitro. J Endocrinol 1974;60:117-25. [PubMed]
- Mitamura K, Nakagawa T, Shimada K, et al. Identification of dehydroepiandrosterone metabolites formed from human prostate homogenate using liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry. J Chromatogr A 2002;961:97-105. [PubMed]
- Acevedo HF, Goldzieher JW. The metabolism of [4-14C] progesterone by hypertrophic and carcinomatous human prostate tissue. Biochim Biophys Acta 1965;111:294-8. [PubMed]
- Di Silverio F, Gagliardi V, Sorcini G, et al. Biosynthesis and metabolism of androgenic hormones in cancer of the prostate. Invest Urol 1976;13:286-8. [PubMed]
- Klein H, Molwitz T, Bartsch W. Steroid sulfate sulfatase in human benign prostatic hyperplasia: characterization and quantification of the enzyme in epithelium and stroma. J Steroid Biochem 1989;33:195-200. [PubMed]
- Koh E, Kanaya J, Namiki M. Adrenal steroids in human prostatic cancer cell lines. Arch Androl 2001;46:117-25. [PubMed]
- Mostaghel EA, Solomon KR, Pelton K, et al. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One 2012;7:e30062. [PubMed]
- Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447-54. [PubMed]
- Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 2010;70:390-400. [PubMed]
- Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 2008;68:6407-15. [PubMed]
- Lubik AA, Gunter JH, Hendy SC, et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res 2011;71:5754-64. [PubMed]
- Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol 2008;295:115-20. [PubMed]
- Locke JA, Wasan KM, Nelson CC, et al. Androgen-mediated cholesterol metabolism in LNCaP and PC-3 cell lines is regulated through two different isoforms of acyl-coenzyme A:Cholesterol Acyltransferase (ACAT). Prostate 2008;68:20-33. [PubMed]
- Locke JA, Nelson CC, Adomat HH, et al. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol 2009;115:126-36. [PubMed]
- Jeong CW, Yoon CY, Jeong SJ, et al. Limited expression of cytochrome p450 17alpha-hydroxylase/17,20-lyase in prostate cancer cell lines. Korean J Urol 2011;52:494-7. [PubMed]
- Kumagai J, Hofland J, Erkens-Schulze S, et al. Intratumoral conversion of adrenal androgen precursors drives androgen receptor-activated cell growth in prostate cancer more potently than de novo steroidogenesis. Prostate 2013;73:1636-50. [PubMed]
- Chun JY, Nadiminty N, Dutt S, et al. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res 2009;15:4815-22. [PubMed]
- Lubik AA, Gunter JH, Hollier BG, et al. IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocr Relat Cancer 2013;20:173-86. [PubMed]
- Liu SV, Schally AV, Hawes D, et al. Expression of receptors for luteinizing hormone-releasing hormone (LH-RH) in prostate cancers following therapy with LH-RH agonists. Clin Cancer Res 2010;16:4675-80. [PubMed]
- Pinski JK, Xiong S, Wang Q, et al. Effect of luteinizing hormone on the steroid biosynthesis pathway in prostate cancer. 2010 Genitourinary Cancers Symposium.
- Arnold JT, Gray NE, Jacobowitz K, et al. Human prostate stromal cells stimulate increased PSA production in DHEA-treated prostate cancer epithelial cells. J Steroid Biochem Mol Biol 2008;111:240-6. [PubMed]
- Mizokami A, Koh E, Izumi K, et al. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr Relat Cancer 2009;16:1139-55. [PubMed]
- Sillat T, Pöllänen R, Lopes JR, et al. Intracrine androgenic apparatus in human bone marrow stromal cells. J Cell Mol Med 2009;13:3296-302. [PubMed]
- Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol 2010;28:1481-8. [PubMed]
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008;26:4563-71. [PubMed]
- Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011;71:6503-13. [PubMed]
- Evaul K, Li R, Papari-Zareei M, et al. 3beta-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology 2010;151:3514-20. [PubMed]
- Li R, Evaul K, Sharma KK, et al. Abiraterone inhibits 3beta-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin Cancer Res 2012;18:3571-9. [PubMed]
- Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res 2012;72:2176-82. [PubMed]


