Medical treatment of male infertility
Introduction
About 15% of couples are infertile and male factor infertility contributes to about 50% of the infertility cases (1). The majority of male infertility is idiopathic, which indicates that the patient has unexplained abnormalities in sperm parameters, or unexplained azoospermia. However, there are multiple known causes of male infertility, and several have a pharmacologic option as the first line of treatment. The medical treatment of known causes of male infertility tend to have targeted and high success rates. In cases of idiopathic or genetic causes of male infertility, the medical management tends to be empirical and is directed for the purposes of optimization.
It is important to appreciate that testicular function involves both the production of testosterone (T) and spermatogenesis, and this function is highly regulated by the hypothalamic-pituitary-gonadal (HPG) axis. Spermatogenesis is dependent on high levels of intratesticular T and follicle-stimulating hormone (FSH) stimulation of the Sertoli cells (2). Despite the requirement for T for spermatogenesis, the administration of T and other androgens have contraceptive properties; they exhibit a negative feedback on HPG and thus inhibit luteinizing hormone (LH) stimulation of intratesticular T production, as well as FSH stimulation of Sertoli cells, and should be avoided. For most known causes of male infertility, the therapeutic goal is the maintenance of the reproductive axis to increase testicular T. However, in certain men with primary testicular failure or idiopathic male infertility, a specific medical therapy has not been identified, and empiric medical treatments are often used. This review article will focus on the non-surgical treatments currently available for male infertility and review the data on the efficacy of those therapies, the list of medications reviewed are summarized in Table 1.
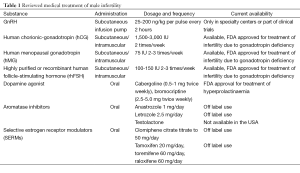
Full table
Hormonal treatment
Gonadotropin-releasing hormone (GnRH)
The pulsatile release of GnRH in the hypothalamus stimulates the release of FSH and LH from the anterior pituitary. In men, normal levels of FSH and LH are responsible for induction of spermatogenesis and maintaining high levels of testicular T (3). Pulsatile administration of GnRH is an effective treatment to replace GnRH deficiency in infertile men with hypogonadotropic hypogonadism (HH) due to a lack of secretion from the hypothalamus (e.g., Kallmann’s syndrome, idiopathic HH). Men with HH have reduced fertility that is usually restored by reestablishing the high intra-testicular T and the FSH stimulation of Sertoli cells (4). The goal of GnRH therapy is to stimulate the release of gonadotropins from the anterior pituitary and subsequent pathways in the HPG (3). The most effective dose for pulsatile GnRH is a dose between 5-20 µg every one to two hours delivered by a subcutaneous pump or needle (5).
GnRH is very effective in inducing spermatogenesis as early as four months after the start of therapy (6). Pulsatile GnRH therapy induces spermatogenesis in about 85% of patients (7,8), and on average 60% of couples will achieve pregnancy after nine months of treatment, and can take up to two years (9). Some men who receive GnRH will see an improvement in their sexual characteristics such as increase in the testicular volume, and other features like pubic hair growth. These changes can be used as clinical markers to monitor treatment. Increase in testicular size, normalization of gonadotropin and T levels, maturation of secondary sexual characteristics, normal baseline inhibin B levels, and absence of cryptorchidism are positive predictors of treatment success (10). When pulsatile GnRH treatment fails to mount a clinical response, the method of administration, effectiveness of the dose, or other causes such as formed anti-GnRH antibodies should be evaluated as part of the differential diagnosis (8). GnRH is successfully used in the treatment of men with HH, but currently there is a lack of evidence to support its use in the treatment of idiopathic infertility. About 10% of patients with HH might not require longtime treatment; there is evidence that the hypothalamus will produce and secret GnRH after treatment is withheld for a short period of time (11). Despite the great outcomes that are experienced in pulsatile GnRH treatment, its use is limited by availability, inconvenience of delivery by carrying a pump, and the need to regularly change subcutaneous needles.
Gonadotropins
The treatment of male infertility in men with pituitary insufficiency (e.g., pituitary adenoma, systemic diseases such as hemochromatosis and sarcoidosis) is based on the use of gonadotropins, therefore spermatogenesis and T production cannot be induced by pulsatile GnRH. Gonadotropins were previously extracted from urine. With advancement in laboratory technology, human chorionic gonadotropin (rec-hCG), FSH (rec-hFSH) and LH (rec-hLH) or highly purified urinary gonadotropins are used with superior quality, activity and performance. There have been no confirmed differences in the safety, purity, or clinical efficacy among the various available highly purified or recombinant gonadotropin products (12).
Initially, hCG is administrated alone. After several months of treatment, if no sperm is detected but adequate serum T levels are achieved, then treatment with FSH is introduced (13). Gonadotropins are self-administered subcutaneous injections with dosages ranging between 75-150 IU of FSH or human menopausal gonadotropin (hMG) two to three times weekly plus 1,500-2,500 IU of hCG twice weekly. The duration of treatment may vary from 6-24 months or more and typically continues until sperm appears in the ejaculate and/or when pregnancy is achieved. Most studies have shown that gonadotropins induce spermatogenesis in approximately 80% of treated men (14), with some demonstrating the use of hCG combined with hFSH, urine-hFSH, or hMG-inducing spermatogenesis in up to 94% of men (15). The time it takes for sperm to appear in the ejaculate varies, most study reporting an average time of seven months and average time of 28 months to achieve pregnancy (16). A multicenter, safety study demonstrated that the combination of hCG and rhFSH induces spermatogenesis in men with HH who failed to respond to treatment with hCG alone (17). The pregnancy rate also varies between studies, 38% to 51% of couple who seeks treatment obtain pregnancy (9,17). The gonadotropins also increased T levels and testicular volume with relatively few side-effects (13). Several factors correlate with the response to treatment. Cryptorchidism, small testicles, elevated BMI, and extreme gonadotropin insufficiency are generally considered a negative prognostic indicator for treatment with gonadotropins (13,18,19). Gonadotropins are generally well tolerated, and proper dose adjustments to optimize T levels will minimize side effects such as gynecomastia, acne, influenza-like symptoms, and weight gain.
Even though GnRH and gonadotropins have proven their benefit and shown success in the induction of spermatogenesis in HH, there is limited evidence for the use of gonadotropins in men with idiopathic infertility. Some of the randomized controlled studies that evaluated the use of hMG or FSH in combination with hCG for the treatment of normogonadotropic oligoasthenoteratozoospermia (OAT) did not show any benefits from treatment (20,21). However, some studies have shown that men with hypospermatogenesis on a fine needle aspiration will have improvement in the seminal parameters and pregnancy rates when treated with gonadotropins compared to other pathology on testicular tissue (22). Currently, there is a lack of consensus on the use of FSH or LH substitute for the treatment of idiopathic OAT and NOA, and since the majority of men with testicular failure have elevated FSH greater than 8.4 IU/liter (23,24), the rationale to administer more gonadotropins is not completely justified. Therefore, these treatments should be considered empirical and more evidence from placebo-controlled trials is required.
Dopamine agonist
For men presenting with infertility and hyperprolactinemia, prolactin-secreting pituitary adenoma (most common functional tumors) should be considered as the underlying cause. Tumors that cause stalk compression and hyperprolactinemia should not be treated with a dopamine agonist. Elevated levels of prolactin inhibit the pulsatile secretion of GnRH, men will present with hypogonadism and infertility, and they might also experience headaches or visual field changes secondary to the pituitary tumor compression. In this setting, dopamine agonists are indicated for the treatment of infertility and the pituitary tumor. Both bromocriptine and cabergoline have been used in the past. However, there is evidence that cabergoline is more effective than bromocriptine in suppressing prolactin production (25), and has been shown to normalize prolactin levels in 70% of bromocriptine-resistant patients (26). Therefore, cabergoline (0.125-1.0 mg twice weekly) is the preferred choice because it has the highest efficacy in normalizing prolactin levels and shrinking prolactin-secreting tumors. Patients that fail to achieve normal prolactin level on maximally tolerated dose, or experience less than 50% reduction in tumor size, and fail to restore fertility most likely have dopamine agonist resistance. The current recommendation for tumors that are resistant to dopamine agonist is to increase the medication dose, or switch from bromocriptine to cabergoline. Patients that fail cabergoline or dose modification are recommended to undergo surgery (27).
Aromatase inhibitor (AI) therapy
AI treatment of men with idiopathic OAT or azoospermia is an off-label use of this medication. AIs (anastrozole 1 mg daily, or letrozole 2.5 mg daily) increase T, decrease estrogen levels, and inhibit the peripheral metabolism of T. The intent is to reduce the estrogenic effect on spermatogenesis. High estrogen levels in combination with low T levels have been shown to impair proper spermatogenesis (28). More importantly, elevated levels of estrogen will lead to feedback inhibition of the HPG axis, and the end result is a decrease in the LH necessary for the production T, and FSH to optimize sperm production (29). The activity of aromatase inhibition regardless of patient BMI suggests that aromatase activity in the Leydig cells is responsible for the T to estradiol (E) conversion and impaired semen parameters (30,31).
A small study of high dose testolactone did not affect semen quality, or pregnancy rates in men with idiopathic oligozoospermia, however T/E ratios were not affected with treatment in this trial (32). The use of testolactone in another trial of men with idiopathic infertility and T <300 ng/dL with T/E ratio <10:1, showed improvement in the hormonal profile and semen parameters (33). These findings suggest that at least a small subset of male infertility patients with elevated serum E levels may benefit from treatment with low dose testolactone. However, there is some evidence that high dose testolactone treatment may actually inhibits T production, so it may not be effective in improving sperm production and fertility. Candidates for aromatase inhibition have usually been identified as men with serum and T <300 ng/dL and T/E ratios >10 (33).
In a non-controlled study comparing anastrozole and testolactone (74 received testolactone, and 104 received anastrozole), in infertile, oligo- or azoospermic men with abnormal T/E ratios, treatment resulted in improvement in sperm concentration, motility, and morphology in all enrolled subjects (30), regardless of which medication the patient was taking. The use of anastrozole might be more effective at increasing the T to estrogen ratio, and is less likely to cause interruption of androgenic steroid production as well as aromatase inhibition as seen with testolactone, and has fewer side effects. The use of the AI letrozole in the treatment of 27 men with oligospermia or azoospermia showed a marked improved in the hormonal profile, and an improvement in the semen parameters including an increase in sperm concentration. Twenty percent of treated oligospermic men achieved spontaneous pregnancy and 24% of azoospermic men had sperm identified in the ejaculate (34). Since most men with low sperm concentration appear to commonly have excess aromatase activity leading to diminished T/E ratio, AIs seem to be effective in restoring LH, FSH, and T levels, improving semen parameters, and re-establishing fertility. Men that benefited the most had the diagnosis of NOA or idiopathic oligoasthenospermia and low T and T/E <10:1 (30,33,35,36). AI therapy is usually well tolerated with rare side effects, including nausea, decreased libido, asymptomatic mild elevation in the liver function tests (37). A very important side effect of AI is the possible decrease of bone mineral density and increase in the total body fat due to the decrease in availability of estrogen (38).
Selective estrogen receptor modulators (SERMs)
SERMs are a class of compounds that act on the estrogen receptor as agonists or antagonists. Before the introduction of intracytoplasmic sperm injection, SERM’s where one of the few option available for men with idiopathic infertility. While SERMs, such as clomiphene citrate (CC), tamoxifen, and toremifene, have been widely used in women for the treatment of breast cancer and osteoporosis, their use in the treatment of male hypogonadism and infertility is currently off-label. CC, like other SERMs, inhibits central estrogen feedback (39) and up regulates the production of LH and FSH, leading to induction of spermatogenesis. Because CC encompasses both a strong intrinsic estrogenic and anti-estrogenic properties, there is concern that the estrogenic effect of clomiphene can potentially have a deleterious effect on spermatogenesis. However, studies have shown that, in hypogonadal men, clomiphene can have substantial positive effects on serum T (40), and can increase pregnancy rates (41). In a case series from multi-international centers, men with non-obstructive azoospermia (n=42) treated with CC dose titrated to serum T level of 600 ng/dL, 64% of patients had sperm in their ejaculate, sufficient for intracytoplasmic sperm injection. However, this study did not have a control group and excluded all Sertoli cell-only syndrome patients (42), indicating that CC might not be effective in all pathological groups of men with infertility.
Tamoxifen citrate and other similar compounds toremifene and raloxifene are non-steroidal estrogen receptor antagonists with a similar mechanism of action as CC at the level of the hypothalamus and pituitary. Randomized controlled trials in men with oligospermia or azoospermia examining the efficacy of tamoxifen (20 mg daily) or toremifene and raloxifene (60 mg daily) have reported improvements in semen parameters and pregnancy rates following three months of treatment (43). However, other studies have shown improvements in the biochemical profile with no effect on semen parameters or fertility outcomes (44).
In a recently published meta-analysis that summarized the latest available randomized controlled trials regarding the use of estrogen antagonists (CC or tamoxifen) as empirical medical therapy for idiopathic male infertility with oligo and/or asthenoteratozoospermia, the pooled data showed that estrogen antagonist use was associated with a statistically significant increase in pregnancy rates compared with controls (OR 2.42; P=0.0004). A significant increase in sperm concentration by a mean difference of 5.24 M (5%; P=0.001) and percent sperm motility by mean difference of 4.55 (P=0.03) were also noted. The same meta-analysis also noted a significant elevation in the serum FSH and T levels that were associated with the use of CC or tamoxifen with no significant difference in adverse events noted between the treated group and controls (45). Other meta-analyses of men with oligoasthenospermia treated with anti-estrogens revealed no or slight increases in the pregnancy rates of 15.4% vs. 12.5% in control subjects. The authors did not support the use of anti-estrogens (46,47).
Antioxidant
Increased rates of infertility have been found in men with seminal fluid containing high levels of reactive oxygen species (ROS) (48). These ROS are associated with sperm dysfunction, germ cell DNA damage with the possibility of impaired fertility, but the exact mechanism is not completely understood. These associations have led clinicians to treat infertile men with antioxidant supplements. A variety of clinical trials have suggested that the use of antioxidant supplements have a slight benefit in improving sperm function and DNA integrity. However, most of these studies are not randomized controlled trials, and to date there are no convincing trials that have demonstrated a significantly higher unassisted pregnancy rate after treating men with antioxidant therapy (49). Moreover, the benefit of antioxidants might be limited to certain groups of patients that is not, as yet, clearly defined. The use of individual antioxidants is very common. These trends have led pharmaceutical companies to produce and market specific combinations of antioxidants, and numerous studies have looked at the benefit of these combinations. A study that looked at the use of vitamin E and C in combination found no improvement in semen parameters or pregnancy rates (50) and a similar study using vitamin E and C found a meaningful reduction in DNA fragmentation but no improvement in the semen parameters when compared to placebo (51). In a randomized controlled trial, the combination of vitamins A, C, E plus NAC and zinc increased sperm concentration with no impact on pregnancy rate (52). This group of patients also had varicocele correction surgery and the increase in sperm concentration can be confounded and not be associated with use of the antioxidant. A systematic review of 17 randomized trials, including 1,665 infertile men, was conducted to evaluate the effects of oral antioxidants (vitamins C and E, zinc, selenium, folate, carnitine and carotenoids) on sperm quality and pregnancy rates in infertile men. Fourteen of the 17 (82%) trials showed an improvement in either sperm quality or pregnancy rate after antioxidant therapy. Ten trials examined pregnancy rate and six showed a significant improvement after antioxidant therapy (53). This systematic review had multiple limitations: most these studies were not controlled and differed in study design. The combined data differed in population, dosage and duration of antioxidants used. Currently there are no specific recommendations on the use of antioxidants in the treatment of male infertility, and the use of these products is completely empirical.
Optimizing surgical sperm extraction with hormonal manipulation
The use of medical therapy to optimize surgical sperm extraction is based also on the concept that spermatogenesis is dependent on high levels of intratesticular T and FSH stimulation of the Sertoli cells (2). Since 60% to 70% of men with NOA will have focal spermatogenesis, the optimization of the hormonal profile in certain patients might be beneficial. The use of CC, AI, and gonadotropins may be beneficial in increasing intratesticular T levels and normalizing estrogen levels prior to sperm retrieval. In a retrospective study, men with KS and NOA who received AIs, clomiphene or hCG before microTESE and experienced T rebound to 250 ng/dL or greater had an increase in the sperm retrieval rate by 22% compared to men who did not reach serum level of 250 ng/dL (54). The same study also showed that KS patients may benefit by specifically using testolactone (54). A similar study that evaluated the use of medical therapy before sperm extraction (CC, AI, and gonadotropin), showed that non-KS men with nonobstructive azoospermia and hypogonadism often respond to hormonal therapy with an increase in T levels, but neither baseline T level nor response to hormonal therapy appears to affect overall sperm retrieval, clinical pregnancy or live birth rates (55). To understand the benefit of pretreatment prior to micro-TESE requires well designed randomized control trials and to date the results are not conclusive. Although there is a paucity of level one evidence, a prospective study that evaluated the use of CC showed a statistically significant increase in favorable testis biopsy patterns and increase in the likelihood of sperm extraction in patients with maturation arrest or hypospermatogenesis on pretreatment biopsy (42). By contrast, 66 men treated with CC for 2-3 months prior to micro-TESE did not exhibit any improvement in sperm retrieval rate or clinical pregnancy rates (55).
The use of gonadotropins to optimize sperm extraction is controversial, and the patient population that might benefit from such treatment is undetermined. Randomized control trials may help to elucidate the role of medical therapy prior to sperm retrieval. A study in men with normal FSH levels and hypospermatogenesis on testicular biopsy suggested possible benefits from such treatment (56). The use of hCG and/or rFSH in men with NOA has been associated with sperm retrieval in men who failed initial micro-TESE who then underwent repeat sperm extraction (57,58), and in KS men before micro-TESE to optimize T levels (54), as well as in men with hypogonadism who failed to normalize their T levels with CC therapy before TESE (59).
Conclusions
Understanding the HPG axis and the effect of estrogen excess is critical for the assessment and treatment of male infertility. However, the goal of infertility treatment in all these men is to optimize LH levels to stimulate T production from the Leydig cells, FSH levels to stimulate Sertoli cells and spermatogenesis, and eliminate any estrogen excess. Pharmacologic therapy is only effective in a handful of known causes of male infertility where the causes are relatively well-defined and understood. Based on current data, hormonal therapies in general should not be used indiscriminately for the treatment of idiopathic male infertility due to questionable efficacy and restrictive cost.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod 1998;13 Suppl 1:33-44. [PubMed]
- Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci 2005;1061:208-20. [PubMed]
- Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med 1991;324:93-103. [PubMed]
- Zitzmann M, Nieschlag E. Hormone substitution in male hypogonadism. Mol Cell Endocrinol 2000;161:73-88. [PubMed]
- Happ J, Ditscheid W, Krause U. Pulsatile gonadotropin-releasing hormone therapy in male patients with Kallmann’s syndrome or constitutional delay of puberty. Fertil Steril 1985;43:599-608. [PubMed]
- Blumenfeld Z, Makler A, Frisch L, et al. Induction of spermatogenesis and fertility in hypogonadotropic azoospermic men by intravenous pulsatile gonadotropin-releasing hormone (GnRH). Gynecol Endocrinol 1988;2:151-64. [PubMed]
- Liu L, Banks SM, Barnes KM, et al. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 1988;67:1140-5. [PubMed]
- Blumenfeld Z, Frisch L, Conn PM. Gonadotropin-releasing hormone (GnRH) antibodies formation in hypogonadotropic azoospermic men treated with pulsatile GnRH--diagnosis and possible alternative treatment. Fertil Steril 1988;50:622-9. [PubMed]
- Büchter D, Behre HM, Kliesch S, et al. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol 1998;139:298-303. [PubMed]
- Pitteloud N, Hayes FJ, Dwyer A, et al. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2002;87:4128-36. [PubMed]
- Raivio T, Falardeau J, Dwyer A, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 2007;357:863-73. [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Birmingham, Alabama. Gonadotropin preparations: past, present, and future perspectives. Fertil Steril 2008;90:S13-20. [PubMed]
- Warne DW, Decosterd G, Okada H, et al. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril 2009;92:594-604. [PubMed]
- Burgués S, Calderón MD. Subcutaneous self-administration of highly purified follicle stimulating hormone and human chorionic gonadotrophin for the treatment of male hypogonadotrophic hypogonadism. Spanish Collaborative Group on Male Hypogonadotropic Hypogonadism. Hum Reprod 1997;12:980-6. [PubMed]
- Miyagawa Y, Tsujimura A, Matsumiya K, et al. Outcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective study. J Urol 2005;173:2072-5. [PubMed]
- Liu PY, Baker HW, Jayadev V, et al. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab 2009;94:801-8. [PubMed]
- Bouloux PM, Nieschlag E, Burger HG, et al. Induction of spermatogenesis by recombinant follicle-stimulating hormone (puregon) in hypogonadotropic azoospermic men who failed to respond to human chorionic gonadotropin alone. J Androl 2003;24:604-11. [PubMed]
- Finkel DM, Phillips JL, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med 1985;313:651-5. [PubMed]
- Burris AS, Rodbard HW, Winters SJ, et al. Gonadotropin therapy in men with isolated hypogonadotropic hypogonadism: the response to human chorionic gonadotropin is predicted by initial testicular size. J Clin Endocrinol Metab 1988;66:1144-51. [PubMed]
- Knuth UA, Hönigl W, Bals-Pratsch M, et al. Treatment of severe oligospermia with human chorionic gonadotropin/human menopausal gonadotropin: a placebo-controlled, double blind trial. J Clin Endocrinol Metab 1987;65:1081-7. [PubMed]
- Kamischke A, Behre HM, Bergmann M, et al. Recombinant human follicle stimulating hormone for treatment of male idiopathic infertility: a randomized, double-blind, placebo-controlled, clinical trial. Hum Reprod 1998;13:596-603. [PubMed]
- Foresta C, Bettella A, Garolla A, et al. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: a prospective, controlled, randomized clinical study. Fertil Steril 2005;84:654-61. [PubMed]
- Sikaris K, McLachlan RI, Kazlauskas R, et al. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab 2005;90:5928-36. [PubMed]
- Gordetsky J, van Wijngaarden E, O’Brien J. Redefining abnormal follicle-stimulating hormone in the male infertility population. BJU Int 2012;110:568-72. [PubMed]
- Webster J, Piscitelli G, Polli A, et al. Dose-dependent suppression of serum prolactin by cabergoline in hyperprolactinaemia: a placebo controlled, double blind, multicentre study. European Multicentre Cabergoline Dose-finding Study Group. Clin Endocrinol (Oxf) 1992;37:534-41. [PubMed]
- Verhelst J, Abs R, Maiter D, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab 1999;84:2518-22. [PubMed]
- Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:273-88. [PubMed]
- Bharti S, Misro MM, Rai U. Clomiphene citrate potentiates the adverse effects of estrogen on rat testis and down-regulates the expression of steroidogenic enzyme genes. Fertil Steril 2013;99:140-8. [PubMed]
- Santen RJ. Feedback control of luteinizing hormone and follicle-stimulating hormone secretion by testosterone and estradiol in men: physiological and clinical implications. Clin Biochem 1981;14:243-51. [PubMed]
- Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol 2002;167:624-9. [PubMed]
- Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril 2012;98:1359-62. [PubMed]
- Clark RV, Sherins RJ. Treatment of men with idiopathic oligozoospermic infertility using the aromatase inhibitor, testolactone. Results of a double-blinded, randomized, placebo-controlled trial with crossover. J Androl 1989;10:240-7. [PubMed]
- Pavlovich CP, King P, Goldstein M, et al. Evidence of a treatable endocrinopathy in infertile men. J Urol 2001;165:837-41. [PubMed]
- Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril 2011;95:809-11. [PubMed]
- Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Clin Pract Endocrinol Metab 2008;4:415-9. [PubMed]
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 2003;52:1126-8. [PubMed]
- Gregoriou O, Bakas P, Grigoriadis C, et al. Changes in hormonal profile and seminal parameters with use of aromatase inhibitors in management of infertile men with low testosterone to estradiol ratios. Fertil Steril 2012;98:48-51. [PubMed]
- Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:1011-22. [PubMed]
- Goldstein SR, Siddhanti S, Ciaccia AV, et al. A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update 2000;6:212-24. [PubMed]
- Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int 2012;110:573-8. [PubMed]
- Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril 2006;86:1664-8. [PubMed]
- Hussein A, Ozgok Y, Ross L, et al. Clomiphene administration for cases of nonobstructive azoospermia: a multicenter study. J Androl 2005;26:787-91. [PubMed]
- Farmakiotis D, Farmakis C, Rousso D, et al. The beneficial effects of toremifene administration on the hypothalamic-pituitary-testicular axis and sperm parameters in men with idiopathic oligozoospermia. Fertil Steril 2007;88:847-53. [PubMed]
- Tsourdi E, Kourtis A, Farmakiotis D, et al. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertil Steril 2009;91:1427-30. [PubMed]
- Chua ME, Escusa KG, Luna S, et al. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013;1:749-57. [PubMed]
- Vandekerckhove P, Lilford R, Vail A, et al. Clomiphene or tamoxifen for idiopathic oligo/asthenospermia. Cochrane Database Syst Rev 2000.CD000151. [PubMed]
- Willets AE, Corbo JM, Brown JN. Clomiphene for the treatment of male infertility. Reprod Sci 2013;20:739-44. [PubMed]
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996;48:835-50. [PubMed]
- Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002;23:737-52. [PubMed]
- Rolf C, Cooper TG, Yeung CH, et al. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod 1999;14:1028-33. [PubMed]
- Greco E, Iacobelli M, Rienzi L, et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 2005;26:349-53. [PubMed]
- Paradiso Galatioto G, Gravina GL, Angelozzi G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol 2008;26:97-102. [PubMed]
- Ross C, Morriss A, Khairy M, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online 2010;20:711-23. [PubMed]
- Ramasamy R, Ricci JA, Palermo GD, et al. Successful fertility treatment for Klinefelter’s syndrome. J Urol 2009;182:1108-13. [PubMed]
- Reifsnyder JE, Ramasamy R, Husseini J, et al. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2012;188:532-6. [PubMed]
- Aydos K, Unlü C, Demirel LC, et al. The effect of pure FSH administration in non-obstructive azoospermic men on testicular sperm retrieval. Eur J Obstet Gynecol Reprod Biol 2003;108:54-8. [PubMed]
- Shiraishi K, Ohmi C, Shimabukuro T, et al. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod 2012;27:331-9. [PubMed]
- Selman H, De Santo M, Sterzik K, et al. Rescue of spermatogenesis arrest in azoospermic men after long-term gonadotropin treatment. Fertil Steril 2006;86:466-8. [PubMed]
- Hussein A, Ozgok Y, Ross L, et al. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int 2013;111:E110-4. [PubMed]


