Surgical management of male infertility: an update
Introduction
Infertility, classically defined as the inability of a couple to conceive after 12 months of intercourse without contraception, affects approximately 15% of the population. Of these couples, 50% of cases are thought to be due to female reproductive factors alone, 30% secondary to male factor alone, and about 20% with a contribution from both partners. Even if an abnormality is found during one partner’s evaluation, both members of an infertile dyad should undergo assessment prior to proceeding with further treatments (1).
Male factor infertility, delineated by abnormal semen analysis according to World Health Organization manual criteria, is thought to affect approximately 7% of the total population (2). The most severe form of infertility, azoospermia—the absence of sperm in at least two separate ejaculated samples with examination of centrifuged samples—affects approximately 1% of the general population (3), and up to 20% of the population who present to an infertility clinic (4).
This article discusses procedures that may improve suboptimal semen parameters, such as varicocele repair, or restore normal fertility potential, such as vasectomy reversal and relief of ejaculatory duct obstruction (EDO). In circumstances when natural conception efforts are not possible, a number of techniques can facilitate the retrieval of sperm for use in assisted reproductive technologies. As such, the surgical management of male infertility has allowed many previously infertile couples to parent their own biological children.
Varicocele
Varicocele refers to dilatation of the pampiniform plexus and is the most common identifiable cause of male factor infertility. Clinical varicoceles are rated on a grading system based on physical examination findings: Grade I, palpable with Valsalva maneuver; Grade II, palpable at rest without Valsalva maneuver; and Grade III, grossly visible. Subclinical varicoceles are detected only via scrotal sonogram (5). They are a common finding with reported ranges in the literature from 8-81% depending on patient population. The seminal study of 275 military men reported clinical varicoceles in 8% of their population (6), but one study of 100 fertile men presenting for vasectomy identified 61% of men with varicocele, 17% of which were clinical (7). A clinical varicocele was reported in 12% of men with normal semen analyses presenting for infertility evaluation, and in 25% of men with abnormal semen parameters (8), while another study population showed that varicocele was present in 35% of men with primary infertility and 81% of men with secondary infertility (9).
Color Doppler ultrasonography (CDUS) is both highly sensitive and specific in the diagnosis of varicocele, measured as maximum venous diameter in the pampiniform plexus with evidence of retrograde flow during Valsalva maneuver (10). It may provide more objective data (venous diameter in mm) than physical exam alone. However, just as physical exam is limited by subjectivity and examiner experience, varicocele assessment via CDUS is also affected by patient position and relaxation, experience of the ultrasonographer, and location of the probe placement. Furthermore, while most physicians agree with a cutoff value of multiple veins of greater than 3 mm diameter with retrograde flow in the diagnosis of varicocele, some suggest that any vein larger than 1 mm is pathologic, while others suggest that only veins larger than 5 mm are clinically significant (11). Currently the Male Infertility Best Practice Policy committee does not routinely recommend CDUS in subfertile patients with suspected varicoceles (12), but it may be a useful adjunct for patients with a difficult physical exam, such as those who are obese, have a small scrotum, or scarring from prior surgery (13).
The most common semen abnormalities associated with a clinical varicocele in men presenting for fertility evaluation include low sperm count (oligospermia), decreased motility (asthenospermia), and/or poor morphology (teratospermia), but as noted above semen parameters may also be normal (14). The evidence regarding subclinical varicoceles is mixed: as previously noted, they are common (up to 61%) with few studies assessing semen quality. The mechanism by which varicoceles may contribute to male factor infertility is not yet well-understood. There are a number of theories based on experimental models, including increased testicular temperature with subsequent negative impact on Sertoli cells and spermatogenesis (15), testicular hypoxia (11), decreases in levels of intratesticular testosterone (16), venous stasis leading to accumulation of toxic metabolites and increased oxidative damage (17), and modifications of the androgen receptor (18). Leydig cell dysfunction due increased testicular temperature may also contribute to hypogonadism (19). While there are multiple etiologic theories supported by some contributory data, there are no conclusive data to date as to why some men present with infertility but the majority of patients with varicocele do not.
The data on whether varicocele repair improves fertility outcomes depend heavily on the initial indication for repair (clinical versus subclinical varicocele, normal versus abnormal semen parameters) and the measured outcome (improvement in semen parameters versus pregnancy and live birth). Furthermore, the heterogeneous data available even in randomized clinical trials and the high dropout rate in these reports can make it difficult to generalize from the conclusions of any one study. A meta-analysis of seventeen studies confirmed that repair of clinical varicoceles in men with abnormal semen analyses improves sperm concentration and motility (14), but outcomes on pregnancy are less clear. Another recent meta-analysis of four randomized controlled trials reporting on pregnancy outcomes after repair of clinical varicoceles in oligospermic men found that while each of the studies individually noted improved pregnancy rates as an outcome, when the treatment population heterogeneity was taken into account the results were not statistically significant (20).
The meta-analysis results and recommendations have varied depending upon which studies were included for meta-analysis. A 2004 Cochrane meta-analysis of eight studies, with pregnancy as the outcome of interest, concluded that there was no evidence that treatment of varicocele improved the chance of conception (21). However, Ficarra et al. noted in 2006 that this meta-analysis included both patients with normal semen analyses and subclinical varicocele. Their reanalysis of the data using only the three studies with patients who had abnormal semen analyses and clinical varicoceles showed a statistically significant difference in pregnancy rates even based on intention-to-treat analysis and a high rate of loss of follow-up after 12 months (36.4% treatment group, 20% control group) (22). The most recent Cochrane meta-analysis of ten studies also included patients with normal semen analyses and subclinical varicocele, but with a planned subgroup analysis of five studies which did not include these patients. Initial and subgroup analyses both suggested that varicocele treatment may improve chance of pregnancy, but they again noted high heterogeneity and suggested the need for further research (23).
The current recommendations regarding treatment of varicocele remain heavily qualified: the Practice Committee of the American Society for Reproductive Medicine recommends repair of varicocele in adolescents with reduced ipsilateral testicular size, and, in the infertile couple attempting to conceive, in adult men with a clinical varicocele, abnormal semen analysis, and a partner with normal or correctible fertility (24). The European Association of Urology 2012 update on male infertility recommends varicocele repair in infertile couples with clinical varicocele, oligospermia, infertility of more than two years duration, and otherwise unexplained infertility (5).
There remains, as noted above, significant controversy over whether repair of subclinical varicocele in a subfertile patient with no other identifiable cause is beneficial; one study noted 41% of patients with improvement in semen parameters postoperatively, but also an equal number with worsening of their semen parameters (25). Repair methods were both surgical and percutaneous embolization. Two randomized clinical trials (26,27) showed improvement in semen parameters but not pregnancy rate, while one (28) showed neither an improvement in semen parameters nor pregnancy rate. However, a more recent small nonrandomized retrospective study (29) showed significant improvement in sperm count and pregnancy rate (12/20) with surgical correction compared to their medical management (19/55) and observation (3/16) groups.
The principle of varicocele repair remains the same regardless of treatment modality: occlusion of veins to eliminate the varicocele, identification and preservation of testicular blood supply, and preservation of the lymphatic vessels to prevent post-procedural hydrocele formation.
Surgery
Surgical treatment remains the mainstay of varicocele repair and can be performed through a number of surgical techniques: (I) open via retroperitoneal, inguinal, or subinguinal approaches; (II) microsurgically through an inguinal or subinguinal incision; (III) laparoscopically using three, two (30), or single-port sites (31); or (IV) robotically, employing either a transperitoneal approach or a subinguinal incision.
Varicocelectomy involves ligation of the aberrantly dilated veins within the spermatic cord while preserving arterial and lymphatic supply and the deferential veins. The site of vein ligation depends on the approach used. For example, if varicocelectomy is performed via an inguinal or subinguinal incision the cremasteric and internal spermatic veins are ligated, whereas if it is performed retroperitoneally the testicular vein is ligated. The open and laparoscopic retroperitoneal techniques may include intentional division of the testicular artery above the internal inguinal ring, relying on collateral arterial inflow to provide blood supply to the testis (10). However, in the inguinal and subinguinal approaches, all encountered arteries are preserved (32).
A large 2009 meta-analysis supports microsurgical varicocelectomy as the gold standard for varicocele repair, with the lowest rate of hydrocele formation (0.4%) and the lowest rate of recurrence (1%) compared to other modalities (33). A more recent comparison of only randomized controlled trials comparing microsurgical varicocelectomy to open and laparoscopic varicocelectomy performed for infertility confirmed these results. Two of the four studies compared all three surgical approaches (open, laparoscopic, and microsurgical) whereas the remaining two studies compared open and microsurgical repairs only. The study found a statistically significant difference in the reduction of hydrocele formation and recurrence in microsurgery compared to laparoscopic and open surgery, with no statistically significant difference in hydrocele or recurrence for laparoscopic versus open surgery (34) (See Table 1). Two small studies demonstrated little difference in outcomes between subinguinal versus inguinal microsurgical varicocelectomies, but showed conflicting results regarding postoperative pain. Shiraishi et al. noted increased scrotal pain with a subinguinal incision (35), while Pan et al. attributed increased pain found in the inguinal group in their study to division of muscle and fascia (36).
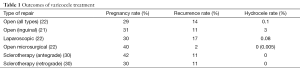
Full table
No studies have compared robotic transperitoneal varicocelectomy to laparoscopic varicocelectomy, and only one report in two patients demonstrates its use in the literature. However, several small studies have studied the use of robot-assisted microsurgical varicocelectomies. Shu et al. performed the pilot study comparing operative time in microsurgical subinguinal varicocelectomy with robotic subinguinal varicocelectomy, and found no difference (37). It is unclear what the indications were for varicocelectomy, and whether operative time took into account setup time for the daVinci® robot system. Semen parameters were not measured. A more recent non-randomized, non-controlled study of 154 patients (chronic orchialgia in 106 pts, including some with oligospermia, and oligo- or azoospermia in 77 pts) found that 77% of the patients with oligospermia and 18% of patients with azoospermia had improvement in semen parameters (38).
Sclerotherapy
Percutaneous retrograde embolization of the gonadal vein for the treatment of varicocele was first described in the 1970s (39). It is less invasive than traditional open retroperitoneal surgery and does not require general anesthesia; for these reasons, some authors have proposed that it be the initial treatment of varicocele (40). It is performed under fluoroscopic guidance with percutaneous access to the spermatic vein obtained in a retrograde fashion via the right femoral vein. It requires significant experience by a vascular interventional radiology team or an appropriately trained urologist, but may allow return to physical activity more quickly than antegrade sclerotherapy approaches or surgical treatments given the lack of incision (41). Current techniques may include alternate venous access sites, such as transjugular and transbrachial approaches, in order to compensate for the difficulty of obtaining access to the spermatic vein and those with complex anatomy (42).
Antegrade sclerotherapy was described in 1988 by Tauber, and can be used either as an initial treatment method or after attempted retrograde sclerotherapy with complex anatomy (41). The antegrade technique can be performed via either inguinal or subinguinal access. After spermatic cord exposure, a single dilated vessel of the pampiniform plexus is exposed and distally ligated. This vein is then cannulated in an antegrade fashion. Drainage to the internal spermatic vein can be confirmed by contrast fluoroscopy. The vein is then sclerosed by antegrade injection of a sclerosing agent and ligated proximally (43). One new technique involves temporary clamping of the spermatic cord proximally prior to injection of the sclerosing agent in order to prevent proximal diffusion and less effective sclerosis (44).
Percutaneous embolization of varicocele is performed with a variety of materials, including angiographic coils (45), venous sclerosis chemical agents, transcatheter foam (46), and more recently, liquid embolic agents (47). The theoretical advantage of foams and liquid agents over the traditional angiographic coils is that they may occlude collateral pathways which, in turn, may translate to decreases in the reported 11% recurrence rate (42).
Complications specific to percutaneous embolization, aside from the risks of infection, contrast reaction and those risks inherent to venous puncture, include phlebitis and migration of embolization materials. Furthermore, a study of radiation exposure in varicocele embolization noted that radiation exposure can be significant: generally low (estimated fatal cancer risk 0.1% in a retrospective series) but in some exceptional cases as high as 100 mSv (estimated risk of fatal radiation-induced cancer 3%) (48). The authors noted that the radiation dose could be substantially reduced with careful technique.
A few studies compare sclerotherapy to open and laparoscopic surgery. May et al. (49) and Beutner et al. (50) both compared laparoscopic surgery to sclerotherapy, and found a higher failure rate (16% vs. 5%) with sclerotherapy, but a higher complication rate with laparoscopy (13-15%). It is important to note that neither study was done in the infertile population, and Beutner’s study included both adults and adolescents. Zucchi et al. compared inguinal varicocelectomy under loupe magnification to antegrade sclerotherapy in patients with abnormal semen parameters and clinical unilateral left varicocele and found a statistically significant improvement in number of motile sperm and fast progressive spermatozoa in the antegrade sclerotherapy group compared to the inguinal varicocelectomy group, with 40% global improvement in semen parameters across both groups and no significant difference in complications or recurrence rates (51). Pregnancy rate was not measured. One small, prospective, randomized study compared the use of retrograde sclerotherapy, antegrade sclerotherapy, and open inguinal varicocelectomy in infertile men, and found improvement in sperm count and total motility across all three groups, with no significant difference in pregnancy rate among the groups (52). Currently, there are no randomized controlled trials comparing sclerotherapy to microsurgery. Given the radiation exposure and lack of superior outcomes to surgery, many urologists reserve sclerotherapy for when other surgical options have failed (53).
Nonobstructive azoospermia (NOA)
NOA—a problem of sperm production with resultant azoospermic ejaculate—can be primary or secondary, congenital or acquired. A large series of 1,583 azoospermic patients found 12% to have no identifiable cause, although this is a lower estimate when compared with prior reports in the literature (54). Known sex chromosome abnormalities formed 21% of this patient population, with Klinefelter’s Syndrome in 14% and Y chromosome microdeletions in 1.7%. Urogenital infections were thought to be the cause of azoospermia in ten percent, with chronic unspecified disease causing seven percent, and malignancy without gonadotoxic treatment constituting six percent.
Correction of endocrinopathies in the uncommon case of hypogonadotropic hypogonadism can result in return of fertility (55), but for most patients NOA is not medically or surgically correctable. Historically, most patients with NOA needed donor insemination or adoption in order to build their families. However, the introduction of intracytoplasmic sperm injection (ICSI) in the early 1990s and the discovery that testicular sperm could be used with in vitro fertilization (IVF) and ICSI to successfully fertilize oocytes (56) changed this. Sperm may be retrieved from men with NOA by standard testicular sperm extraction (TESE), or microdissection testicular sperm extraction (microTESE) (57). One center is now performing robotic-assisted microsurgical TESE, as well.
Open TESE involves a small incision (or multiple incisions) in the tunica albuginea at a location of the surgeon’s choice. The testis is squeezed to extrude tubules, and the biopsy specimen(s) obtained using a pair of surgical scissors. The technique for microTESE is more standardized, as first described by Schlegel: a transverse hemispheric incision in the tunica albuginea allows the surgeon to bivalve the testis and, under 20× to 40× magnification with the operating microscope, one attempts to identify and selectively collect larger, more opaque seminiferous tubules (58). A biopsy specimen is also obtained for histologic analysis.
Numerous recent studies have confirmed the success of microTESE in obtaining sperm compared to traditional TESE. One nonrandomized trial of 133 men noted a 56.9% sperm retrieval rate in microTESE versus a 38.2% success rate with standard TESE (59). One meta-analysis noted four particular subsets of patients who may optimally benefit from microTESE rather than random open biopsy: patients with mosaic or nonmosaic Klinefelter Syndrome, patients with chemotherapy-induced azoospermia, patients with azoospermia after orchidopexy for cryptorchidism, and patients with Y microdeletions in the AZFc region. These populations are thought to represent men with small, limited areas of sperm production in the testes, with sperm found in dilated, opaque seminiferous tubules that can best be identified with the aid of optical magnification (60). One group is currently in the process of developing a nomogram to predict the likelihood of sperm retrieval prior to microdissection given known patient characteristics. Preliminary results suggest that in their nomogram model, the presence of Klinefelter Syndrome or a history of cryptorchidism had the largest modifying effect on successful sperm retrieval, with the contribution of varicocele minor and not statistically significant (61).
There have been several attempts to further refine the microdissection technique given that one of the critiques of the procedure is its long operative time compared to traditional TESE. A recent retrospective study of 900 patients found sperm on initial unilateral microdissection in 474 men, but with only 8% success in finding sperm in the contralateral testis in those who underwent bilateral microdissection for failure to find sperm on initial exploration. They concluded that two specific populations—patients with Klinefelter Syndrome or those with hypospermatogenesis—may benefit from contralateral dissection in the event that unilateral sperm retrieval is unsuccessful (62). One small study of systematic upper, middle, and lower pole biopsies in conjunction with microTESE suggests that the combination may be more successful in retrieving sperm (66.2%) than either technique alone (63).
A contemporary modification of microsurgical technique incorporates the use of robotic assistance. One group has noted that they have performed twelve robotic-assisted microsurgical TESEs, but have not published their outcomes other than to state that the procedure is feasible and there were no complications in their study population (38).
An interesting adjunct to TESE is the role of varicocelectomy then subsequent TESE in the patient with NOA and a clinical varicocele. A recent small observational study of 36 patients examined the timing of varicocelectomy: 19 patients with grade 3 unilateral left varicocele and NOA underwent microsurgical inguinal varicocelectomy three months prior to magnified (loupe) TESE and 16 underwent it at the same time as TESE (64). They showed significant improvement in sperm retrieval rate during TESE with earlier varicocelectomy (57.8% vs. 25%). However, interestingly, in semen analyses six months after TESE, both groups also had sperm present in ejaculated samples (57.8% vs. 37.5%). No semen analysis was done in the interval period prior to TESE in the patients who had undergone previous varicocelectomy. By contrast, Inci retrospectively studied 96 nonrandomized patients with any grade clinical varicocele and NOA, 66 of whom underwent microsurgical inguinal/subinguinal varicocelectomy one year prior to microTESE (65). On the day of microTESE a semen analysis was performed to confirm persistent azoospermia prior to surgical sperm extraction attempt. They found significant improvement in surgical sperm retrieval rate (53% vs. 30%). This was confirmed by Haydardedeoglu et al. (60.8% vs. 38.5%) who also noted an improvement in implantation, clinical pregnancy, and live birth rates in a population of men with NOA and a history of grade 3 varicocele repair (66). In contrast to Zampieri’s study, they found higher pregnancy rates in patients with a shorter interval since varicocelectomy, but the time intervals were much longer (an average of 40 months since prior varicocelectomy in the shorter group). These studies are in contrast to Schlegel’s initial 2004 study, which found equivalent microTESE retrieval rates (60%) between varicocelectomy and nonvaricocelectomy groups; of note, that population included patients with subclinical varicocele (67).
In the twenty years since the advent of using surgically-retrieved sperm for IVF-ICSI, it is hardly surprising that some men may choose to undergo a repeat procedure. One retrospective study of 126 repeat microTESEs after 963 initial successful microTESEs reported a sperm retrieval rate of 82%. In their study population, the pregnancy rate after initial microTESE was 42%, and after repeat microTESE was 39% (68). Another retrospective study of 216 patients who had had prior TESE (40 with successful microTESE, 72 with successful TESE, and 104 with unsuccessful TESE) showed an 81% success rate in patients with NOA and successful prior TESE, but only a 27% success rate in patients with NOA and a history of unsuccessful initial TESE (69).
Neither TESE nor microTESE are risk-free, and both come with risks of bleeding, hematoma, infection, and intratesticular scar formation, as well as excessive harvest of testicular tissue leading to hypogonadism. Serum testosterone levels after microTESE may decrease to 80% of baseline at 3-6 months, but recover to 95% by 18 months (70). The longer-term effects of TESE, microdissection or standard, on testicular histology and spermatogenesis are unknown.
Obstructive azoospermia (OA)
OA—a blockage of the reproductive tract leading to absence of sperm from the ejaculate—is less common than NOA, with studies reporting rates of 11-40% (5,54,71). The mainstay of treatment is surgical management via sperm extraction or restoration of outflow of the reproductive tract via reconstruction or alleviation of blockage. The etiologies of OA may be congenital [e.g., congenital bilateral absence of the vasa deferentia (CBAVD)] or acquired, as in vasectomy, scarring caused by previous infection, or iatrogenic injury from prior inguinal surgeries.
Ejaculatory duct obstruction (EDO)
EDO is rare: there are few recent studies describing its prevalence, but older studies suggest that it occurs in less than 5% of men with OA (72). The classic presentation of EDO is low-volume, acidic ejaculate with oligo- or azoospermia, a normal hormonal profile, and palpable vasa deferentia. Imaging findings suggest dilated seminal vesicles (SVs), prostatic cysts or calcifications, or dilated ejaculatory ducts on transrectal ultrasound (TRUS) (73). However, patient presentation may vary considerably, as they may present with functional or partial obstruction rather than complete obstruction, and with complaints unrelated to fertility such as pain or dysuria (74).
Multiple studies of imaging modalities have been conducted, primarily with small numbers of patients due to the rarity of the disease. TRUS is cheap, convenient, and does not require an incision. It is effective in the diagnosis of dilated SVs, but this finding is neither sensitive nor specific to EDO. Given the constraints of the rectal probe, TRUS may be limited in its ability to localize the level of obstruction (75). Purohit et al. (76) compared the use of three invasive measures in 25 patients (8 of whom were infertile) suspected of having EDO both clinically and on TRUS: (I) SV sperm aspiration; (II) seminal vesiculography using a 30-gauge spinal needle placed under TRUS guidance and contrast patterns confirmed by fluoroscopy; and (III) chromotubation of the ejaculatory ducts through transrectal injection of methylene blue into the SVs and visual confirmation of obstruction with no dye efflux noted during urethroscopy. The authors hypothesized that appropriate patient selection through dynamic imaging (such as vesiculography and chromotubation) may improve outcomes after surgical management, but it is important to note that in their study population, only the patients who had positive dynamic imaging findings progressed to surgical management.
One recent study of the use of magnetic resonance imaging (MRI) in the diagnosis of EDO in 18 patients identified ejaculatory duct cysts in five patients, unilateral or bilateral ejaculatory duct dilatation in nine patients, and Müllerian duct cysts in four patients. These findings were confirmed at surgery and the authors concluded that MRI, in allowing more accurate determination of the location, degree, and cause of obstruction, could help facilitate preoperative planning regarding the depth of resection needed to clear the obstruction (77).
The classic treatment for EDO, transurethral resection of the ejaculatory ducts (TURED), was first described in 1973. Using a 24 French cystourethroscope and an electrocautery resectoscope loop, the urethra is resected in the midline over the proximal verumontanum for bilateral obstruction or more laterally for unilateral obstruction, with successful resection determined by visualization of fluid expression intraoperatively from the ejaculatory ducts. Indigo carmine may be instilled under TRUS guidance into the SVs to observe for efflux of blue dye from the opened ejaculatory ducts for confirmation of patency. The most common complications of TURED include hematuria and epididymoorchitis (78).
Newer technologies are being applied to the transurethral management of EDO. Bipolar electrocautery was used in 42 infertile patients with azoospermia or severe oligospermia due to EDO. The investigators used pure cutting current with no electrocautery to perform the resection and confirmed relief of obstruction through prostate massage to express seminal fluid under cystoscopic visualization. Of the azoospermic patients, 60% had return of sperm to the ejaculate. In the whole study cohort, 38% of patients had return to normal semen parameters, with a 31% pregnancy rate at 18-month follow-up (79). Lee et al. described the use of the holmium: YAG laser in combination with monopolar TUR in a case report of a patient with a midline prostatic cyst. They elected to use the laser, with its much smaller diameter, due to the degree of prostatic urethral narrowing imposed by this cyst. They unroofed the cyst with the laser, and then completed the resection via monopolar TUR (80).
Other recently-described techniques for relief of EDO include direct ejaculatory duct recanalization using retrograde balloon dilation (81) or retrograde insertion of 6F/6.5F vesiculoscopes (82,83). However, the use of these techniques cannot necessarily be generalized to the infertile population as study sizes are very small, indications for treatment are heterogeneous, and semen analyses were not performed in the majority of these study populations. Nonetheless, preliminary results are encouraging and bear further investigation.
Vasal obstruction
The most common cause of OA is vasectomy. In a recent sampling of more than 10,000 men ages 15-45 through the National Survey for Family Growth, approximately 7% of men reported having had a vasectomy in their lifetime, but nearly 20% of that population stated that they desire future children (84). The basic steps of vasectomy reversal involve excision of the obstructed segment of vas, microscopic assessment of fluid from the testicular vasal segment to confirm presence of sperm or other features reassuring for patency, and confirmation of patency of the abdominal vasal segment. If both proximal and distal patency is confirmed, the freshly-cut ends of the vas are then reapproximated to complete vasovasostomy (VV). If secondary epididymal obstruction is suspected, vasoepididymostomy (VE) is warranted (85).
Currently, vasectomy reversal is most commonly performed microsurgically, although it has been performed without the aid of the microscope and recent studies explore the utility of robotic-assistance. The principles of a successful anastomosis include mucosa-to-mucosa apposition, a tension-free and watertight reconstruction, and preservation of the blood supply. There are patient-related qualities that affect the success of a vasectomy reversal aside from the procedure type chosen: a 1991 multicenter retrospective study of 1,469 microsurgical vasectomy reversals noted improved patency and pregnancy rates with shorter interval since vasectomy and when sperm could be aspirated from the testicular end of the vas (86). Most microsurgical series report patency rates of 85-98% (using variable definitions of patency) and live birth rates of 38-84% (87).
The quality of vasal fluid from the testicular segment is a marker of proximal patency: thick, creamy fluid with no sperm or no fluid can signify secondary epididymal obstruction. In these cases, VE—end-to-side anastomosis of the vas to a patent epididymal tubule—is indicated (85). Patency and live birth rates are lower than in VV (70-90% and 32-56%, respectively) due to technical difficulty and possible epididymal dysfunction secondary to pressure-related changes and inflammation. VE is more often necessary with longer duration of obstruction (87-89).
Open single-layer spatulated VV was first described in 1919, and in the subsequent historic literature a variety of suture materials and temporary stents to aid in visualization of the vasal lumen were employed. The reported success rates were up to 60%, with success not being clearly defined (90). The use of the operating microscope was introduced in the 1970s (91).
Early animal studies in the 1980s exploring the use of fibrin glue for a sutureless or few-suture technique were promising, including macroscopic rabbit studies with fibrin glue and temporary splints (92) and microscopic rat studies using fibrin glue alone (93) or fibrin glue combined with two or three sutures (94). By contrast, another rat model showed that a biomaterial wrap improved operating time without compromising patency. Patency rates in the sealant group were 70% compared with a 92% patency rate in the biomaterial wrap group (95). One human study using Tisseel® with three bolstering sutures in 42 patients reported nine pregnancies in 21 patients actively trying to conceive, and suggested that the procedure was less time-consuming and technically easier compared to microsurgical or open vasectomy reversal (96). However, the study has several flaws that make it difficult to recommend this technique, including a high failure rate and very low rates of follow-up.
The microsurgical approach can be performed with one, two, or three layers (97). The advantages of a three-layer technique over the two- or one-layer techniques include the ability to bring markedly discrepant luminal diameters together with prevention of dog-ears and a more watertight anastomosis, but operative time is longer and the procedure is more technically difficult. In 1998, Goldstein et al. described the microdot technique in effort to reduce degree of technical difficulty of the procedure. A total of six evenly-spaced microdots are placed on the cut ends of the vas using a pen in order to mark the needle exit points. This pre-placement planning allows for more precise and evenly spaced suture placement (98).
There have been no studies comparing the three-layer closure to the one- or two-layer reapproximations. A single retrospective study compared the modified one-layer approach to the two-layer approach in a single institution and found no difference in patency postoperatively (99), but characteristics of the testicular vasal fluid were not measured, and neither were postprocedural pregnancy rates. One-layer and two-layer outcome comparisons had been previously studied by the Vasovasostomy Study Group in 1991, with the same results (86).
There are no randomized controlled trials comparing open to microsurgical vasectomy reversal; most studies are single-institution retrospective reviews. Given the lack of head-to-head comparative studies, critics of the microsurgical technique note that operative time is significantly increased and there is an unclear benefit in pregnancy outcomes. One small, recent, retrospective study suggests that in the hands of a surgeon experienced with both modalities, a single-layer macroscopic approach can provide decreased operative time and cost without compromising results (100).
The first animal studies on robot-assisted VV were performed in 2004 (101) with the first human study published by Parekattil et al. in 2010 (102). They compared 110 two-layer robotic-assisted reversals (performed for either fertility or chronic orchialgia after vasectomy) to 45 standard two-layer microsurgical reversals. VV or VE was performed as clinically indicated for both approaches. They found a significant increase in patency rates, defined as greater than one million sperm/ejaculated sample, for robotic versus microsurgical procedures (96% vs. 80% at 17-month follow-up) but no significant difference in pregnancy rates at one year (65% vs. 55%). They also noted that their initial operative duration was significantly longer for robotic compared to microsurgical procedures, but median operative time was significantly decreased (103). However, the reported operative duration did not take into account the extra 30-60 minutes needed for robotic setup and preparation at the beginning of the case.
Although vasectomy reversal is expensive, a number of studies have shown via various modeling methods that vasectomy reversal may be more cost-effective than sperm retrieval performed in conjunction with IVF-ICSI (104-106). Complications are rare, with scrotal hematoma among the most common. Rate of return of sperm to the ejaculate is variable, with slower return of sperm with VE (67). Late failure is also possible. One study of 823 patients who had return of sperm to the ejaculate after VV noted reocclusion in 1% (97).
VV may also be used as a treatment approach for repair of iatrogenic vasal obstruction. The vas deferens may be damaged by inadvertent intraoperative transection or compression during inguinal surgery. The incidence of injury varies between 0.3% and 7.2% in adult hernia repair, but is reported in as high as 27% in patients with a history of pediatric inguinal hernia repair (107). It may be associated with injury to the ipsilateral blood supply and a long obstructive interval. A 1998 study of 36 procedures noted that microsurgical repair of iatrogenic vasal injury is both possible and successful (108). Crossed VV or VE, with anastomosis to the contralateral side for patent outflow, is indicated in three circumstances: (I) large vasal defects where an ipsilateral tension-free anastomosis is not possible; (II) unilateral inguinal vasal obstruction with a contralateral obstructed or atrophic testis; or (III) unilateral epididymal obstruction with a contralateral atrophic testis.
Sperm retrieval
Sperm retrieval methods provide a final common pathway for patients with OA who either elect not to undergo reconstruction, have failed reconstruction, or have anatomy not conducive to reconstruction. Harvest techniques include aspiration of sperm from the epididymis (percutaneously or open with or without the aid of the microscope) or the testis (percutaneous or open).
Percutaneous epididymal sperm aspiration (PESA) involves percutaneous cannulation of the epididymis with a small-gauge needle and aspiration of the epididymal fluid. PESA has the advantage of being technically easy and does not require an operating room, general anesthetic, or microsurgical training (109). The largest study of PESA to date is a retrospective study of 255 patients undergoing PESA for OA of various etiologies, including CBAVD, vasectomy, failed VV, and iatrogenic injury to the vas (110). The investigators were successful in obtaining abundant motile sperm in 75% of cases, rare motile sperm in 9%, nonmotile sperm in 11%, and no sperm in 5%. A total of 19% of patients proceeded to TESE techniques, with mature spermatozoa found in all of these patients. The authors observed a significant need for progression to testicular sperm retrieval in older patients and patients with smaller testicular volumes.
Microsurgical epididymal sperm aspiration (MESA) was first described in 1994 in the setting of a patient with CBAVD, and involves exposure of the epididymis through a small incision with isolation and puncture of individual epididymal tubules to aspirate fluid (111). It has the advantage of allowing direct identification of the tubules, which can be particularly advantageous in the setting of extensive scarring or proximal obstruction. It can also be performed after failed PESA. However, there have been no studies directly comparing the use of MESA and PESA, and very few studies other than the initial studies (109,112) studying the rate of sperm retrieval in MESA.
When epididymal sperm extraction is unsuccessful or if the practitioner prefers, a patient may proceed to TESE for use with ICSI via either percutaneous or open methods (113-115). Percutaneous needle aspiration has been performed with various numbers of passes and needle gauges, with some evidence that a larger 18- or 19-gauge needle may be more successful in obtaining sperm than a 21-gauge needle (116). There are very limited, older studies detailing the use of percutaneous testicular biopsy with a biopsy gun and, although the studies have noted increased yield and consistent preservation of the testicular architecture with a biopsy gun compared to a needle biopsy, they have also reported avascular areas of the testes after gun biopsy on ultrasound, thought to be the result of small arterial rupture (117).
Percutaneous approaches to OA are usually successful in obtaining sperm sufficient for use in ICSI, with most studies noting upwards of 95% success rate for either epididymal or testicular extraction of sperm (118). One study found that sperm recovery in TESE was 100% with ICSI fertilization rates of 66% and live delivery rates of 62% (119). A cohort of 1,121 men with OA who had undergone either epididymal or TESE for ICSI found that the source and etiology of the obstruction did not affect fertilization or pregnancy rate (120).
Conclusions
In an era of assisted reproductive technology, microsurgery, and robotic surgery, the surgical management of the infertile male is both complex and encouraging, offering biologic paternity to men who historically would have had to resort to adoption or donor sperm in order to parent.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: This article does not contain any studies with human or animal subjects performed by either of the authors.
References
- Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab 2013;98:3532-42. [PubMed]
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011;25:271-85. [PubMed]
- Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo) 2013;68 Suppl 1:27-34. [PubMed]
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol 1989;142:62-5. [PubMed]
- Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol 2012;62:324-32. [PubMed]
- Clarke BG. Incidence of varicocele in normal men and among men of different ages. JAMA 1966;198:1121-2. [PubMed]
- Kursh ED. What is the incidence of varicocele in a fertile population? Fertil Steril 1987;48:510-1. [PubMed]
- The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril 1992;57:1289-93. [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [PubMed]
- Trum JW, Gubler FM, Laan R, et al. The value of palpation, varicoscreen contact thermography and colour Doppler ultrasound in the diagnosis of varicocele. Hum Reprod 1996;11:1232-5. [PubMed]
- Lee J, Binsaleh S, Lo K, et al. Varicoceles: the diagnostic dilemma. J Androl 2008;29:143-6. [PubMed]
- Male Infertility Best Practice Policy Committee of the American Urological Association, Practice Committee of the American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril 2004;82:S142-5. [PubMed]
- Stahl P, Schlegel PN. Standardization and documentation of varicocele evaluation. Curr Opin Urol 2011;21:500-5. [PubMed]
- Agarwal A, Deepinder F, Cocuzza M, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology 2007;70:532-8. [PubMed]
- Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol 2012;19:538-50. [PubMed]
- Ozturk MI, Koca O, Keles MO, et al. The impact of unilateral experimental rat varicocele model on testicular histopathology, leydig cell counts, and intratesticular testosterone levels of both testes. Urol J 2013;10:973-80. [PubMed]
- Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatoz oa from infertile men with varicocele. Fertil Steril 2003;80:1431-6. [PubMed]
- Soares TS, Fernandes SA, Lima ML, et al. Experimental varicocoele in rats affects mechanisms that control expression and function of the androgen receptor. Andrology 2013;1:670-81. [PubMed]
- Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol 1989;142:743-5. [PubMed]
- Baazeem A, Belzile E, Ciampi A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol 2011;60:796-808. [PubMed]
- Evers JL, Collins JA. Surgery or embolisation for varicocele in subfertile men. Cochrane Database Syst Rev 2004.CD000479. [PubMed]
- Ficarra V, Cerruto MA, Liguori G, et al. Treatment of varicocele in subfertile men: The Cochrane Review--a contrary opinion. Eur Urol 2006;49:258-63. [PubMed]
- Kroese AC, de Lange NM, Collins J, et al. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev 2012;10:CD000479. [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril 2008;90:S247-9. [PubMed]
- Jarow JP, Ogle SR, Eskew LA. Seminal improvement following repair of ultrasound detected subclinical varicoceles. J Urol 1996;155:1287-90. [PubMed]
- Yamamoto M, Hibi H, Hirata Y, et al. Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. J Urol 1996;155:1636-8. [PubMed]
- Unal D, Yeni E, Verit A, et al. Clomiphene citrate versus varicocelectomy in treatment of subclinical varicocele: a prospective randomized study. Int J Urol 2001;8:227-30. [PubMed]
- Grasso M, Lania C, Castelli M, et al. Low-grade left varicocele in patients over 30 years old: the effect of spermatic vein ligation on fertility. BJU Int 2000;85:305-7. [PubMed]
- Seo JT, Kim KT, Moon MH, et al. The significance of microsurgical varicocelectomy in the treatment of subclinical varicocele. Fertil Steril 2010;93:1907-10. [PubMed]
- Link BA, Kruska J, Wong C, et al. Two trocar laparoscopic varicocelectomy: approach and outcomes. JSLS 2006;10:151-4. [PubMed]
- Kaouk JH, Palmer JS. Single-port laparoscopic surgery: initial experience in children for varicocelectomy. BJU Int 2008;102:97-9. [PubMed]
- Mirilas P, Mentessidou A. Microsurgical subinguinal varicocelectomy in children, adolescents, and adults: surgical anatomy and anatomically justified technique. J Androl 2012;33:338-49. [PubMed]
- Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl 2009;30:33-40. [PubMed]
- Ding H, Tian J, Du W, et al. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int 2012;110:1536-42. [PubMed]
- Shiraishi K, Oka S, Ito H, et al. Comparison of the results and complications of retroperitoneal, microsurgical subinguinal, and high inguinal approaches in the treatment of varicoceles. J Androl 2012;33:1387-93. [PubMed]
- Pan F, Pan L, Zhang A, et al. Comparison of two approaches in microsurgical varicocelectomy in Chinese infertile males. Urol Int 2013;90:443-8. [PubMed]
- Shu T, Taghechian S, Wang R, et al. Initial experience with robot-assisted varicocelectomy. Asian J Androl 2008;10:146-8. [PubMed]
- Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl 2013;15:67-74. [PubMed]
- Lima SS, Castro MP, Costa OF. A new method for the treatment of varicocele. Andrologia 1978;10:103-6. [PubMed]
- Shlansky-Goldberg RD, VanArsdalen KN, Rutter CM, et al. Percutaneous varicocele embolization versus surgical ligation for the treatment of infertility: changes in seminal variables and pregnancy outcomes. J Vasc Interv Radiol 1997;8:759-67. [PubMed]
- Chan P. Management options of varicoceles. Indian J Urol 2011;27:65-73. [PubMed]
- Iaccarino V, Venetucci P. Interventional radiology of male varicocele: current status. Cardiovasc Intervent Radiol 2012;35:1263-80. [PubMed]
- Tauber R, Pfeiffer D. Surgical atlas varicocele: antegrade scrotal sclerotherapy. BJU Int 2006;98:1333-44. [PubMed]
- Mancini M, Carmignani L, Agarwal A, et al. Antegrade subinguinal sclerotization with temporary clamping of the spermatic cord: a new surgical technique for varicocele. Urology 2011;77:223-6. [PubMed]
- Storm DW, Hogan MJ, Jayanthi VR. Initial experience with percutaneous selective embolization: a truly minimally invasive treatment of the adolescent varicocele with no risk of hydrocele development. J Pediatr Urol 2010;6:567-71. [PubMed]
- Li L, Zeng XQ, Li YH. Safety and effectiveness of transcatheter foam sclerotherapy for testicular varicocele with a fluoroscopic tracing technique. J Vasc Interv Radiol 2010;21:824-8. [PubMed]
- Urbano J, Cabrera M, Alonso-Burgos A. Sclerosis and varicocele embolization with N-butyl cyanoacrylate: experience in 41 patients. Acta Radiol 2014;55:179-85. [PubMed]
- Chalmers N, Hufton AP, Jackson RW, et al. Radiation risk estimation in varicocele embolization. Br J Radiol 2000;73:293-7. [PubMed]
- May M, Johannsen M, Beutner S, et al. Laparoscopic surgery versus antegrade scrotal sclerotherapy: retrospective comparison of two different approaches for varicocele treatment. Eur Urol 2006;49:384-7. [PubMed]
- Beutner S, May M, Hoschke B, et al. Treatment of varicocele with reference to age: a retrospective comparison of three minimally invasive procedures. Surg Endosc 2007;21:61-5. [PubMed]
- Zucchi A, Mearini L, Mearini E, et al. Treatment of varicocele: randomized prospective study on open surgery versus Tauber antegrade sclerotherapy. J Androl 2005;26:328-32. [PubMed]
- Fayez A, El Shantaly KM, Abbas M, et al. Comparison of inguinal approach, scrotal sclerotherapy and subinguinal antegrade sclerotherapy in varicocele treatment: a randomized prospective study. Urol Int 2010;85:200-3. [PubMed]
- Robinson SP, Hampton LJ, Koo HP. Treatment strategy for the adolescent varicocele. Urol Clin North Am 2010;37:269-78. [PubMed]
- Tüttelmann F, Werny F, Cooper TG, et al. Clinical experience with azoospermia: aetiology and chances for spermatozoa detection upon biopsy. Int J Androl 2011;34:291-8. [PubMed]
- Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68 Suppl 1:81-8. [PubMed]
- Devroey P, Liu J, Nagy Z, et al. Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril 1994;62:639-41. [PubMed]
- Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl 2012;14:109-15. [PubMed]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131-5. [PubMed]
- Ghalayini IF, Al-Ghazo MA, Hani OB, et al. Clinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermia. J Clin Med Res 2011;3:124-31. [PubMed]
- Schlegel PN. Nonobstructive azoospermia: a revolutionary surgical approach and results. Semin Reprod Med 2009;27:165-70. [PubMed]
- Ramasamy R, Padilla WO, Osterberg EC, et al. A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2013;189:638-42. [PubMed]
- Ramasamy R, Reifsnyder JE, Husseini J, et al. Localization of sperm during microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2013;189:643-6. [PubMed]
- Marconi M, Keudel A, Diemer T, et al. Combined trifocal and microsurgical testicular sperm extraction is the best technique for testicular sperm retrieval in “low-chance” nonobstructive azoospermia. Eur Urol 2012;62:713-9. [PubMed]
- Zampieri N, Bosaro L, Costantini C, et al. Relationship between testicular sperm extraction and varicocelectomy in patients with varicocele and nonobstructive azoospermia. Urology 2013;82:74-7. [PubMed]
- Inci K, Hascicek M, Kara O, et al. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol 2009;182:1500-5. [PubMed]
- Haydardedeoglu B, Turunc T, Kilicdag EB, et al. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: a retrospective pilot study. Urology 2010;75:83-6. [PubMed]
- Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril 2004;81:1585-8. [PubMed]
- Ramasamy R, Ricci JA, Leung RA, et al. Successful repeat microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2011;185:1027-31. [PubMed]
- Hussein A. Evaluation of diagnostic testis biopsy and the repetition of testicular sperm extraction surgeries in infertility patients. Fertil Steril 2013;100:88-93. [PubMed]
- Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology 2005;65:1190-4. [PubMed]
- Tanrikut C, Goldstein M. Obstructive azoospermia: a microsurgical success story. Semin Reprod Med 2009;27:159-64. [PubMed]
- Pryor JP, Hendry WF. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertil Steril 1991;56:725-30. [PubMed]
- Carter SS, Shinohara K, Lipshultz LI. Transrectal ultrasonography in disorders of the seminal vesicles and ejaculatory ducts. Urol Clin North Am 1989;16:773-90. [PubMed]
- Nagler HM, Rotman M, Zoltan E, et al. The natural history of partial ejaculatory duct obstruction. J Urol 2002;167:253-4. [PubMed]
- Engin G, Kadioğlu A, Orhan I, et al. Transrectal US and endorectal MR imaging in partial and complete obstruction of the seminal duct system. A comparative study. Acta Radiol 2000;41:288-95. [PubMed]
- Purohit RS, Wu DS, Shinohara K, et al. A prospective comparison of 3 diagnostic methods to evaluate ejaculatory duct obstruction. J Urol 2004;171:232-5. [PubMed]
- Guo Y, Liu G, Yang D, et al. Role of MRI in assessment of ejaculatory duct obstruction. J Xray Sci Technol 2013;21:141-6. [PubMed]
- McQuaid JW, Tanrikut C. Ejaculatory duct obstruction: current diagnosis and treatment. Curr Urol Rep 2013;14:291-7. [PubMed]
- Tu XA, Zhuang JT, Zhao L, et al. Transurethral bipolar plasma kinetic resection of ejaculatory duct for treatment of ejaculatory duct obstruction. J Xray Sci Technol 2013;21:293-302. [PubMed]
- Lee JY, Diaz RR, Choi YD, et al. Hybrid method of transurethral resection of ejaculatory ducts using holmium:yttriumaluminium garnet laser on complete ejaculatory duct obstruction. Yonsei Med J 2013;54:1062-5. [PubMed]
- Lawler LP, Cosin O, Jarow JP, et al. Transrectal US-guided seminal vesiculography and ejaculatory duct recanalization and balloon dilation for treatment of chronic pelvic pain. J Vasc Interv Radiol 2006;17:169-73. [PubMed]
- Wang H, Ye H, Xu C, et al. Transurethral seminal vesiculoscopy using a 6F vesiculoscope for ejaculatory duct obstruction: initial experience. J Androl 2012;33:637-43. [PubMed]
- Liu ZY, Sun YH, Xu CL, et al. Transurethral seminal vesiculoscopy in the diagnosis and treatment of persistent or recurrent hemospermia: a single-institution experience. Asian J Androl 2009;11:566-70. [PubMed]
- Sharma V, Le BV, Sheth KR, et al. Vasectomy demographics and postvasectomy desire for future children: results from a contemporary national survey. Fertil Steril 2013;99:1880-5. [PubMed]
- Herrel L, Hsiao W. Microsurgical vasovasostomy. Asian J Androl 2013;15:44-8. [PubMed]
- Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 1991;145:505-11. [PubMed]
- Lee R, Li PS, Schlegel PN, et al. Reassessing reconstruction in the management of obstructive azoospermia: reconstruction or sperm acquisition? Urol Clin North Am 2008;35:289-301. [PubMed]
- Shridharani A, Sandlow JI. Vasectomy reversal versus IVF with sperm retrieval: which is better? Curr Opin Urol 2010;20:503-9. [PubMed]
- Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int 2005;96:598-601. [PubMed]
- Fenster H, McLoughlin MG. Vasovasostomy--is the microscope necessary? Urology 1981;18:60-4. [PubMed]
- Silber SJ. Microscopic technique for reversal of vasectomy. Surg Gynecol Obstet 1976;143:631. [PubMed]
- Bach D, Distelmaier W, Weissbach L. Animal experiments on reanastomosis of the vas deferens using fibrin glue. Urol Res 1980;8:29. [PubMed]
- Silverstein JI, Mellinger BC. Fibrin glue vasal anastomosis compared to conventional sutured vasovasostomy in the rat. J Urol 1991;145:1288-91. [PubMed]
- Vankemmel O, de la Taille A, Rigot JM, et al. Vasal reanastomosis using fibrin glue combined with sutures: which combination of sutures in a delayed protocol? Experimental study in rats. Eur Urol 1998;33:318-22. [PubMed]
- Schiff J, Li PS, Goldstein M. Toward a sutureless vasovasostomy: use of biomaterials and surgical sealants in a rodent vasovasostomy model. J Urol 2004;172:1192-5. [PubMed]
- Ho KL, Witte MN, Bird ET, et al. Fibrin glue assisted 3-suture vasovasostomy. J Urol 2005;174:1360-3. [PubMed]
- Schwarzer JU. Vasectomy reversal using a microsurgical three-layer technique: one surgeon’s experience over 18 years with 1300 patients. Int J Androl 2012;35:706-13. [PubMed]
- Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol 1998;159:188-90. [PubMed]
- Fischer MA, Grantmyre JE. Comparison of modified one- and two-layer microsurgical vasovasostomy. BJU Int 2000;85:1085-8. [PubMed]
- Safarinejad MR, Lashkari MH, Asgari SA, et al. Comparison of macroscopic one-layer over number 1 nylon suture vasovasostomy with the standard two-layer microsurgical procedure. Hum Fertil (Camb) 2013;16:194-9. [PubMed]
- Schiff J, Li PS, Goldstein M. Robotic microsurgical vasovasostomy and vasoepididymostomy: a prospective randomized study in a rat model. J Urol 2004;171:1720-5. [PubMed]
- Parekattil SJ, Atalah HN, Cohen MS. Video technique for human robot-assisted microsurgical vasovasostomy. J Endourol 2010;24:511-4. [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt J, et al. Robotic assisted versus pure microsurgical vasectomy reversal: technique and prospective database control trial. J Reconstr Microsurg 2012;28:435-44. [PubMed]
- Garceau L, Henderson J, Davis LJ, et al. Economic implications of assisted reproductive techniques: a systematic review. Hum Reprod 2002;17:3090-109. [PubMed]
- Pasqualotto FF, Lucon AM, Sobreiro BP, et al. The best infertility treatment for vasectomized men: assisted reproduction or vasectomy reversal? Rev Hosp Clin Fac Med Sao Paulo 2004;59:312-5. [PubMed]
- Lee R, Li PS, Goldstein M, et al. A decision analysis of treatments for obstructive azoospermia. Hum Reprod 2008;23:2043-9. [PubMed]
- Tekatli H, Schouten N, van Dalen T, et al. Mechanism, assessment, and incidence of male infertility after inguinal hernia surgery: a review of the preclinical and clinical literature. Am J Surg 2012;204:503-9. [PubMed]
- Sheynkin YR, Hendin BN, Schlegel PN, et al. Microsurgical repair of iatrogenic injury to the vas deferens. J Urol 1998;159:139-41. [PubMed]
- Craft IL, Khalifa Y, Boulos A, et al. Factors influencing the outcome of in-vitro fertilization with percutaneous aspirated epididymal spermatozoa and intracytoplasmic sperm injection in azoospermic men. Hum Reprod 1995;10:1791-4. [PubMed]
- Yafi FA, Zini A. Percutaneous epididymal sperm aspiration for men with obstructive azoospermia: predictors of successful sperm retrieval. Urology 2013;82:341-4. [PubMed]
- Tournaye H, Devroey P, Liu J, et al. Microsurgical epididymal sperm aspiration and intracytoplasmic sperm injection: a new effective approach to infertility as a result of congenital bilateral absence of the vas deferens. Fertil Steril 1994;61:1045-51. [PubMed]
- Nudell DM, Conaghan J, Pedersen RA, et al. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod 1998;13:1260-5. [PubMed]
- Craft I, Bennett V, Nicholson N. Fertilising ability of testicular spermatozoa. Lancet 1993;342:864. [PubMed]
- Schoysman R, Vanderzwalmen P, Nijs M, et al. Pregnancy after fertilisation with human testicular spermatozoa. Lancet 1993;342:1237. [PubMed]
- Devroey P, Liu J, Nagy Z, et al. Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril 1994;62:639-41. [PubMed]
- Carpi A, Menchini Fabris FG, Todeschini G, et al. Large-needle percutaneous aspiration biopsy of the testicle in men with non obstructive azoospermia. Fertil Steril 2006;86:464-5. [PubMed]
- Tuuri T, Moilanen J, Kaukoranta S, et al. Testicular biopty gun needle biopsy in collecting spermatozoa for intracytoplasmic injection, cryopreservation and histology. Hum Reprod 1999;14:1274-8. [PubMed]
- Esteves SC, Lee W, Benjamin DJ, et al. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol 2013;189:232-7. [PubMed]
- Omurtag K, Cooper A, Bullock A, et al. Sperm recovery and IVF after testicular sperm extraction (TESE): effect of male diagnosis and use of off-site surgical centers on sperm recovery and IVF. PLoS One 2013;8:e69838. [PubMed]
- Miyaoka R, Esteves SC. Predictive factors for sperm retrieval and sperm injection outcomes in obstructive azoospermia: do etiology, retrieval techniques and gamete source play a role? Clinics (Sao Paulo) 2013;68 Suppl 1:111-9. [PubMed]


