Application of Raman spectroscopy in Andrology: non-invasive analysis of tissue and single cell
Introduction
The famous quote by Sydney Brenner “Progress in science relies on new techniques, new discoveries and new ideas, probably in that order” implies the significance of introducing new technology or research tools for scientific progress (1). For instance, X-ray for body scanning, advent of endoscopic surgery, microarrays for DNA sequencing analysis. Raman spectroscopy is an optical technology that relies on the principle of inelastic scattering between light photons with biological tissues. When photons of light interact with the molecules in materials, different light-scattering patterns arise. In most cases, the photons emitted hold the energy or wavelength unchanged as the incident light (= Rayleigh scattering), but occasionally their donate or receive energy due to the molecular interactions, the process known as inelastic scattering or “Raman shift” (2). In 1930, the Indian physician C.V. Raman was awarded the Nobel Prize for his contribution to discover the Raman affect that lays the foundation of Raman spectroscopy (3).
The Raman frequency shifts are conventionally measured in wavelength (cm–1) (4), depending on the atomic mass or molecular bonds of specimens, all the chemical information involved is presented on a Raman spectrum that can be further interpreted and analyzed using statistical, chemical, and morphological methods. Raman enables subtle analysis of molecular structure and biological compositions, and as a non-invasive, non-destructive and even non-contact detection method, it has been employed in various medical fields. Today, there are four main types of Raman spectroscopy being used, including resonance Raman spectroscopy (RRS), coherent anti-Stokes Raman spectroscopy (CARS), stimulated Raman spectroscopy (SRS) and surface enhanced Raman scattering (SERS) (5). Recently, a new technique called Raman optical tweezer emerges, it combines Raman spectroscopy with optical tweezer, which benefits the trap and analysis of micrometer-size particles (6). The Raman optical tweezer levitates the trapped cells above the substate to reduce the fluorescence effects and the Brownian motion from untrapped cells.
Application of Raman spectroscopy in human studies is marked by its capacity to distinguish normal and pathological tissues. Skin is one of the most human tissues studied at the early age of Raman spectroscopy, Emma et al. [1977] first examined the skin lesions and compared its Raman spectra with that of normal skin (7). And in the ensuing years, numerous promising results were witnessed, including detection of different parts of in vivo and in vitro skin, identification of skin pigment melanin, or distinguishment of skin cancers (8-11). Raman spectroscopy also contribute to the diagnosis and even prognostic evaluation of cancers in breast, lung, stomach, cervix uteri, brain, larynx, etc. (12-17).
In this review, we summarized the advance about the utility of Raman spectroscopy in Andrology, articles pertaining to the mechanism of Raman spectroscopy and its applications were gathered from PubMed and Ovid database using MeSH terms associated with prostate, testis, seminal plasma and single sperm cell (Figure 1). We also reported on the feasibility of Raman spectroscopy in real-time clinical practice.
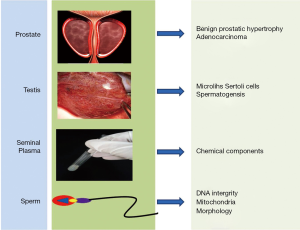
Applications in prostate and testis
Prostate
Special interest in the use of Raman spectroscopy in Urology emerges recently. It has been used in vitro to detect renal and bladder cancers, identify renal lithiasis, study different layers of bladder wall, and distinguish malignant cells in urinary system (18-21). In an article by Amos Shapiro and his colleagues, the Raman system successfully discovered a distinct peak at 1,584 cm–1 wave shift that separate benign and different grades of malignent bladder tissues, with an excellent sensitivity of 92% and specificity of 91% (22). Here, we just focus on the field of Andrology, the prostate and testis.
In prostate, Raman spectroscopy is mostly used to differentiate benign prostatic hypertrophy (BPH) and prostate adenocarcinoma (CaP). Crow et al. were the first to record the Raman signal of prostatic tissue, and successfully discovered the variations in glycogen and nucleic acid contents between BPH and CaP (23). The promising results propelled them to further examined three different grades of adenocarcinoma as classified by Gleason Score—GS <7, GS =7, and GS >7 (24). By constructing a diagnostic algorithm, the Raman spectroscopy was able to distinguish each pathological type with an overall accuracy of 89%. Due to its high sensitivity and specificity, they suggested that Raman spectroscopy has some potentials to guide prostate biopsy in vivo and help with determination of tumor resection margins during radical prostatectomy. A meaningful study using Raman spectroscopy was conducted by Patel et al. [2011] (25), who compared the benign prostatic tissue between populations of high-risk (UK) and low-risk (India) of CaP, attempted to figure out the biological foundations for their different susceptibilities to diseases. The results indicated the secondary protein structure variations (involving Amide I/II—1,582, 1,551, 1,667, 1,541 cm–1 in glandular epithelium; Amide I/II—1,663, 1,624, 1,761, 1,782, 1,497 cm–1 for adjacent stroma) as pivotal biomolecular markers segregating the two cohorts. Raman spectroscopy was also employed to study the relationship between spectral signal of Gleason 7 prostate cancers and their prognostic after radical prostatectomy, as well as the diagnosis and prognosis of castration-resistant prostate cancer (26).
Different parts of prostate were also examined, respectively. Patel and Martin [2010] used Raman spectroscopy to interrogate the normal, cancer-free prostate zones, tried to explore the underlying reasons of zone-specific susceptibility to pathology, as CaP mainly arises in PZ, prostatic hypertrophy (BPH) arises in TZ, while CZ has a relative immunity to diseases (27). The results highlighted 781 cm–1 (cytosine/uracil) and 787 cm–1 (DNA) as the key factors differentiating PZ from TZ and CZ epithelia; and identified 1,459 cm–1 (lipids and proteins) and 1,003 cm–1 (phenylalanine) as the determinant features discriminating TZ with CZ epithelia. The authors supposed that the increased amounts of nucleic acid in PZ were probably due to differential gene expression profiles or DNA adduct formation that contain phase I/II metabolising enzymes capable of inducing carcinogens; while the elevated levels of proteins and lipids in TZ could be explained as the variations of hormones like testosterone, dihydrotestosterone or epidermal growth factors leading to BPH and TZ CaP. In that study, they also examined the stromal zones against glandular elements, as characterized by protein/lipid (1,459 and 1,100 cm–1) and less DNA/RNA (781 and 787 cm–1).
PNT1A (immortalized normal prostate cell line) and LNCaP (malignant cell line derived from prostate metastases) were mapped using Raman spectroscopy by Taleb et al. (28). Applying spectral processing methods of partial least-squares discriminant analyses (PLSDAs) and adjacent band ratios (ABRs), the two cell lines were perfectly distinguished from each other, positions indicating to A-DNA/RNA conformation (711, 813, 1,100, 1,243, and 1,572 cm–1) were mainly found in LNCaP, while bands corresponding to B-DNA conformation (733, 798, and 1,091 cm–1) were presented more intensified in PNT1A. Crow et al. compared two well-differentiated, androgen-sensitive cell lines (LNCaP and PCa 2b) with two poorly differentiated, androgen-insensitive cell lines (DU145 and PC 3), demonstrated that Raman was able to identify CaP samples of varying biological aggressiveness with an overall sensitivity of 98% and a specificity of 99% (29). Other works in this area involved the detection of complexed forms of prostate specific antigen (PSA) from blood sample using SERS, the low-level detection of biomarker could be used for diagnosis and early-stage prediction of prostate cancer (30).
Testis
In comparison to other tissues, the use of Raman spectroscopy has lagged behind for interrogation of testicular function and constitution, this is probably due to the scarce sheer number of testicular samples available. De Jong et al. [2004] first reported the research about testis, he performed Raman mapping of frozen sections of testicular microlithiasis, and demonstrated that the microliths were composed of hydroxyapatite and were located within the seminiferous tubules (31). And when microliths were found surrounded by glycogen, the testicular tissues were usually associated with malignant germ-cell neoplasms. Maybe the Raman spectroscopy could also be used to evaluate intact human seminiferous tubules, once the spectral differences between tubules of complete and incomplete spermatogenesis could be found, the Raman spectroscopy would help to guide Microdissection testicular sperm extration (Micro-TESE), which means only the tubules matching the signal of complete spermatogenesis shall be removed.
Raman spectroscopic analysis of seminal plasma and sperm
Seminal plasma
Actually, Raman spectroscopic analysis of seminal plasma was initially carried out for forensic purposes rather than concerns in male fertility, since precise determination and identification of body fluid at a crime scene would certainly benefit forensic investigations (32-34). Lednev et al. reported three principal components that satisfactorily represented Raman spectra of semen, involving tyrosine, proteins (amide I/III, albumin, choline) and spermine phosphate hexahydrate (SPH). The combination of the three spectral components can be used to identify any unknown fluids to be semen, and even semen from different species could be differentiated, for instance, the signal of peaks at 536, 829, and 1,452 cm–1 were more stronger in human semen than that of canine, and the 1,342 and 1,004 cm–1 peaks were found exclusively in human sample. But, there were no significant differences in the Raman spectra of semen acquired from various human donors.
Since seminal plasma is composed of the fluids gathered from seminal vesicles, prostate, and other reproductive glands, it provides the nutritive and protective environment for sperm and has some relationships with semen quality. Huang et al. examined the Raman spectra of seminal plasma from normal and abnormal semen samples, and found the two could be clearly distinguished according to the peak ratios between 1,449 and 1,418 cm–1 (35). And the left-handed polarized SERS spectroscopy delivered the best diagnostic result with a sensitivity of 95.8% and specificity of 64.9% (36).
Sperm
Kubasek et al. [1986] evaluated the DNA of salmon sperm and found it had a B-type conformation very similar to that of a synthesized model B-DNA oligomer (37). This is the first attempt to examine the sperm by Raman spectroscopy. Since then, however, no other Raman investigations of sperm were ever reported until very recently scientists tried to find a non-invasive method to evaluate the human sperm.
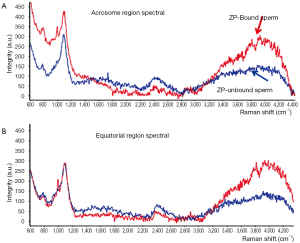
Several approaches have been described regarding to the study of human sperm by Raman spectroscopy (38-40), the semen samples were generally washed and smeared directly on quartz coverslips, and then detected by Raman spectroscopy, except for only one case in which the sperm membranes and tails were removed for the concern of obtaining spectra of pure nuclear DNA-protein complex (41). Though these studies reported some similar results, fundamental discrepancies existed. Huser et al. reported the 785 cm–1 peak strength was indicative of the efficiency of DNA packaging process, and the variations in the ratio of 785/1,092 cm–1 or 1,442/1,092 cm–1 could be employed to discriminate sperm of normal, pear, small and double head, a relationship that was not found in other studies. Besides, the Raman spectra in this study showed almost excellent similarities in tail, mid-section and acrosomal region, a phenomenon which was repeated in only one article by our team who used Raman spectroscopy to distinguish sperm that can bind to zona pellucida (40) (Figure 2). We found two regions (800-900 and 3,200-4,000 cm–1) shifted high at the acrosome region of the ZP-bound sperm compared with unbound sperm. Since ZP naturally select sperm of normal DNA integrity and morphology, thus the ZP-bound sperm are more likely to fertilize the oocytes than ZP-unbound sperm. Using Raman spectroscopy to distinguish ZP-bound sperm could in theory enhance the outcomes of ICSI. In this article, we also described a novel scanning method for single sperm cell according to its anatomic structure (Figure 3). In other studies, however, distinct Raman spectra of different parts of sperm were demonstrated, for example, Meister et al. found the sperm nucleus was dominated by nucleic acid bands around 788 cm–1, and the middle piece was represented by the breathing mode of the adenine and guanine bases near 1,575 cm–1. Even concensus was failed to reach on as to the signal of same regions of sperm between studies, for example, C. Mallidis et al. described a unique 1,092 cm–1 peak in distal head segment, indicating the PO4 backbone of DNA. Reasons for those discrepancies were probably due to different Raman systems being used, including different laser wavelengths, power, and scanning time. And the Raman system should be calibrated for every single time before its usage. Fortunately, the Raman spectroscopy has been demonstrated to successfully detect sperm DNA damage, mitochondrial status, and fertilization potential. And our group also used it to distinguish sertoli cells from testes of obstructive azoospermia and non-obstructive azoospermia (42).
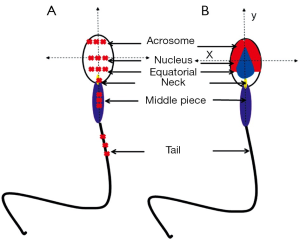
Except for single point analysis, applying confocal Raman microspectroscopy (CRM) that combines Raman spectroscopy with a confocal optical microscope, chemical map of single sperm cell could be constructed with location of same feature being assigned to a specific color (38,39). Raman image enabled unambiguously identification of different parts of sperm, even very small irregularities could be distinguished, such as vacuoles in the sperm head. In a latest study by Huang et al. (43), with a self-written image analysis system the Raman spectroscopy was able to identify normal sperm with both morphological and biochemical information, and the intensity ratio of 1,055 to 1,095 cm–1 indicated a potential biomarker for assessing the sperm DNA integrity.
Further directions
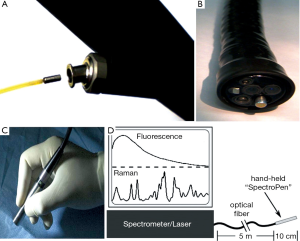
Further application of Raman spectroscopy as a feasible clinical tool still needs additional improvements in this technology. Considering the sheer size of Raman spectroscopy, specially designed optical fiber probes need to be developed for the purpose of intraoperative assistance. The probes should be able to be incorporated into catheters, laparoscopes, endoscopes, or cannulas. Surprisingly, progress has been made, Huang et al. developed a Raman endoscopy system by inserting a 1.8 mm Raman endoscopic probe into the working channel of an endoscope during gastroscopy, with which he gathered a total of 2,748 in vivo gastric tissue spectra (2,465 normal and 283 cancer) from 305 patients, and developed a diagnostic algorithm with a predictive accuracy of 80.0%, sensitivity of 90.0% and specificity of 73.3% (44). He also applied this technique to assess Raman spectral properties of nasopharyngeal and laryngeal tissues through transnasal, image-guided Raman endoscopy (17) (Figure 4A,B). Mohs et al. developed a hand-held spectroscopic pen device called SpectroPen (45) (Figure 4C,D). During in vivo studies using mice model bearing breast tumors, the pen device was able to precisely detect the tumor margins preoperatively and intraoperatively, and was demonstrated to have almost the same signal-to-noise ratio, spectral resolution, and accuracy as the routine Raman spectroscopy. Also, in the manuscript by Mosier-Boss et al., two commercially available, portable Raman systems were evaluated (46).
Serious issue has to do with the safety of Raman spectroscopy. Though many studies described Raman laser as a non-invasive detection method, almost none of them examined its safety, such as DNA impairments caused by Raman laser toward tissue or cells. Taking sperm for example, our aim of Raman study on single sperm cell is to distinguish morphological normal and DNA integral sperm for infertile patients and use it for intracytoplasmic sperm injection (ICSI), if Raman spectroscopy damages the sperm DNA integrity or causes other subtle changes in chromosome structure leading to fertilization failure, abortion, or unpredictable congenital diseases of infants, we should be blamed by our conscience. Thus, additional investigations are required before its final application in clinic.
Finally, it is often hard to set up standard criterions for signal of Raman spectra, just like the discrepancies in the studies of sperm. The outcomes of Raman scanning are vulnerable to various external influences, including the working parameters of Raman system, methods by which samples are prepared, even the room temperature and moisture around the Raman spectrometer would exert influences on the outcomes.
Though there are limitations that need to be addressed seriously, Raman spectroscopy still has great potentials to serve as a clinical tool for real-time scanning of living tissues in vivo and for fast diagnosis of diseases. Use of Raman spectroscopy avoids unnecessary biopsies and delivers immediate pathological diagnosis in real-time intraoperative cancer resections. And in the future, the Raman spectroscopy could have some potentials to guide testicular biopsy, help to raise the sperm retrieval rates and narrow down postoperative complications.
Conclusions
As a novel technique, Raman spectroscopy provides fast, convenient and non-invasive interrogations of biological tissues, and reveals the changes in molecular structure and chemical components. Application of Raman spectroscopy for real-time detection of living tissues and diagnosis of diseases provides potential intraoperative assistance, but still needs a lot of improvements in the technique and strict safety investigations. Future research in Raman spectroscopy may herald a new era for Andrology.
Acknowledgements
Funding: Supported by Science and Technology Commission of Shanghai Municipality (No: 10JCI409900), and National Natural Science Foundation of China (Key Program: 31230048).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Huang WE, Li M, Jarvis RM, et al. Shining light on the microbial world the application of Raman microspectroscopy. Adv Appl Microbiol 2010;70:153-86. [PubMed]
- Brauchle E, Schenke-Layland K. Raman spectroscopy in biomedicine - non-invasive in vitro analysis of cells and extracellular matrix components in tissues. Biotechnol J 2013;8:288-97. [PubMed]
- Rao AR, Hanchanale V, Javle P, et al. Spectroscopic view of life and work of the Nobel Laureate Sir C.V. Raman. J Endourol 2007;21:8-11. [PubMed]
- Hanlon EB, Manoharan R, Koo TW, et al. Prospects for in vivo Raman spectroscopy. Phys Med Biol 2000;45:R1-59. [PubMed]
- Li M, Xu J, Romero-Gonzalez M, et al. Single cell Raman spectroscopy for cell sorting and imaging. Curr Opin Biotechnol 2012;23:56-63. [PubMed]
- Snook RD, Harvey TJ, Correia Faria E, et al. Raman tweezers and their application to the study of singly trapped eukaryotic cells. Integr Biol (Camb) 2009;1:43-52. [PubMed]
- Emma EL, Howell GM, Adrian CW, et al. Applications of Raman spectroscopy to skin Research. Skin Res Technol 1997;3:147-53.
- Caspers PJ, Lucassen GW, Wolthuis R, et al. In vitro and in vivo Raman spectroscopy of human skin. Biospectroscopy 1998;4:S31-9. [PubMed]
- Huang Z, Lui H, Chen XK, et al. Raman spectroscopy of in vivo cutaneous melanin. J Biomed Opt 2004;9:1198-205. [PubMed]
- Lui H, Zhao J, McLean D, et al. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res 2012;72:2491-500. [PubMed]
- Mittal R, Balu M, Krasieva T, et al. Evaluation of stimulated Raman scattering microscopy for identifying squamous cell carcinoma in human skin. Lasers Surg Med 2013;45:496-502. [PubMed]
- Damayanti NP, Fang Y, Parikh MR, et al. Differentiation of cancer cells in two-dimensional and three-dimensional breast cancer models by Raman spectroscopy. J Biomed Opt 2013;18:117008. [PubMed]
- Oshima Y, Shinzawa H, Takenaka T, et al. Discrimination analysis of human lung cancer cells associated with histological type and malignancy using Raman spectroscopy. J Biomed Opt 2010;15:017009. [PubMed]
- Teh SK, Zheng W, Ho KY, et al. Diagnostic potential of near-infrared Raman spectroscopy in the stomach: differentiating dysplasia from normal tissue. Br J Cancer 2008;98:457-65. [PubMed]
- Vargis E, Kanter EM, Majumder SK, et al. Effect of normal variations on disease classification of Raman spectra from cervical tissue. Analyst 2011;136:2981-7. [PubMed]
- Aguiar RP, Silveira L Jr, Falcão ET, et al. Discriminating neoplastic and normal brain tissues in vitro through Raman spectroscopy: a principal components analysis classification model. Photomed Laser Surg 2013;31:595-604. [PubMed]
- Bergholt MS, Lin K, Zheng W, et al. In vivo, real-time, transnasal, image-guided Raman endoscopy: defining spectral properties in the nasopharynx and larynx. J Biomed Opt 2012;17:077002. [PubMed]
- Barman I, Dingari NC, Singh GP, et al. Selective sampling using confocal Raman spectroscopy provides enhanced specificity for urinary bladder cancer diagnosis. Anal Bioanal Chem 2012;404:3091-9. [PubMed]
- Zhuang Z, Li N, Guo Z, et al. Study of molecule variations in renal tumor based on confocal micro-Raman spectroscopy. J Biomed Opt 2013;18:31103. [PubMed]
- Sudlow K, Woolf A. Identification of renal calculi by their Raman spectra. Clin Chim Acta 1991;203:387-93. [PubMed]
- de Jong BW, Bakker Schut TC, Wolffenbuttel KP, et al. Identification of bladder wall layers by Raman spectroscopy. J Urol 2002;168:1771-8. [PubMed]
- Shapiro A, Gofrit ON, Pizov G, et al. Raman molecular imaging: a novel spectroscopic technique for diagnosis of bladder cancer in urine specimens. Eur Urol 2011;59:106-12. [PubMed]
- Crow P, Kendall C, Wright M, et al. Evaluation of Raman spectroscopy to provide a real time, optical method for discrimination between normal and abnormal tissue in the prostate. Eur Urol 2002;1:80.
- Crow P, Stone N, Kendall CA, et al. The use of Raman spectroscopy to identify and grade prostatic adenocarcinoma in vitro. Br J Cancer 2003;89:106-8. [PubMed]
- Patel II, Trevisan J, Singh PB, et al. Segregation of human prostate tissues classified high-risk (UK) versus low-risk (India) for adenocarcinoma using Fourier-transform infrared or Raman microspectroscopy coupled with discriminant analysis. Anal Bioanal Chem 2011;401:969-82. [PubMed]
- Wang L, He D, Zeng J, et al. Raman spectroscopy, a potential tool in diagnosis and prognosis of castration-resistant prostate cancer. J Biomed Opt 2013;18:87001. [PubMed]
- Patel II, Martin FL. Discrimination of zone-specific spectral signatures in normal human prostate using Raman spectroscopy. Analyst 2010;135:3060-9. [PubMed]
- Taleb A, Diamond J, McGarvey JJ, et al. Raman microscopy for the chemometric analysis of tumor cells. J Phys Chem B 2006;110:19625-31. [PubMed]
- Crow P, Barrass B, Kendall C, et al. The use of Raman spectroscopy to differentiate between different prostatic adenocarcinoma cell lines. Br J Cancer 2005;92:2166-70. [PubMed]
- Grubisha DS, Lipert RJ, Park HY, et al. Femtomolar detection of prostate-specific antigen: an immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal Chem 2003;75:5936-43. [PubMed]
- De Jong BW, De Gouveia Brazao CA, Stoop H, et al. Raman spectroscopic analysis identifies testicular microlithiasis as intratubular hydroxyapatite. J Urol 2004;171:92-6. [PubMed]
- Virkler K, Lednev IK. Raman spectroscopy offers great potential for the nondestructive confirmatory identification of body fluids. Forensic Sci Int 2008;181:e1-5. [PubMed]
- Virkler K, Lednev IK. Raman spectroscopic signature of semen and its potential application to forensic body fluid identification. Forensic Sci Int 2009;193:56-62. [PubMed]
- Sikirzhytski V, Sikirzhytskaya A, Lednev IK. Advanced statistical analysis of Raman spectroscopic data for the identification of body fluid traces: semen and blood mixtures. Forensic Sci Int 2012;222:259-65. [PubMed]
- Huang Z, Chen X, Chen Y, et al. Raman spectroscopic characterization and differentiation of seminal plasma. J Biomed Opt 2011;16:110501. [PubMed]
- Chen X, Huang Z, Feng S, et al. Analysis and differentiation of seminal plasma via polarized SERS spectroscopy. Int J Nanomedicine 2012;7:6115-21. [PubMed]
- Kubasek WL, Wang Y, Thomas GA, et al. Raman spectra of the model B-DNA oligomer d(CGCGAATTCGCG)2 and of the DNA in living salmon sperm show that both have very similar B-type conformations. Biochemistry 1986;25:7440-5. [PubMed]
- Mallidis C, Wistuba J, Bleisteiner B, et al. In situ visualization of damaged DNA in human sperm by Raman microspectroscopy. Hum Reprod 2011;26:1641-9. [PubMed]
- Meister K, Schmidt DA, Bründermann E, et al. Confocal Raman microspectroscopy as an analytical tool to assess the mitochondrial status in human spermatozoa. Analyst 2010;135:1370-4. [PubMed]
- Liu F, Zhu Y, Liu Y, et al. Real-time Raman microspectroscopy scanning of the single live sperm bound to human zona pellucida. Fertil Steril 2013;99:684-689.e4.
- Sánchez V, Redmann K, Wistuba J, et al. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil Steril 2012;98:1124-9.e1-3.
- Ma M, Yang S, Zhang Z, et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum Reprod 2013;28:1863-73. [PubMed]
- Huang Z, Chen G, Chen X, et al. Rapid and label-free identification of normal spermatozoa based on image analysis and micro-Raman spectroscopy. J Biophotonics 2014;7:671-5. [PubMed]
- Duraipandian S, Sylvest Bergholt M, Zheng W, et al. Real-time Raman spectroscopy for in vivo, online gastric cancer diagnosis during clinical endoscopic examination. J Biomed Opt 2012;17:081418. [PubMed]
- Mohs AM, Mancini MC, Singhal S, et al. Hand-held spectroscopic device for in vivo and intraoperative tumor detection: contrast enhancement, detection sensitivity, and tissue penetration. Anal Chem 2010;82:9058-65. [PubMed]
- Mosier-Boss PA, Putnam MD. The evaluation of two commercially available, portable Raman systems. Anal Chem Insights 2013;8:83-97. [PubMed]


