Distal urethroplasty for fossa navicularis and meatal strictures
Introduction
Urethral strictures are a source of significant morbidity for adult males in all age groups and are associated with considerable health care spending (1). The reported incidence in the literature is as high as 0.6%, translating into to approximately 5,000 inpatient admissions and 1.5 million outpatient visits annually in the United States alone. For each individual diagnosed with urethral stricture disease, there is an annual increase in health care expenditures of more than $6,000, even after controlling for medical co-morbidities (1). Optimizing the approach to the management of strictures can therefore significantly impact upon both quality of life for individual patients and healthcare resource utilization as a whole. The management of urethral stricture disease must account for the heterogeneous nature of strictures, which present distinctive therapeutic challenges based on their underlying etiology and specific anatomic location. Distal urethral strictures confined to the meatus and fossa navicularis are particularly challenging because: (I) consideration must be given not only to establishment of durable patency of the urethra but also maintenance of glans cosmesis; and (II) these strictures are frequently related to lichen sclerosus, an inflammatory process which can cause local tissue destruction and a propensity for disease recurrence following treatment. Based on these distinctive characteristics, distal urethral strictures are best managed by an individualized, patient-centered approach. Herein we will review the available therapeutic approaches and reported outcomes for treatment of distal urethral strictures in an effort to help clarify decision making for the treating urologist faced with this nuanced reconstructive challenge.
Incidence and etiology
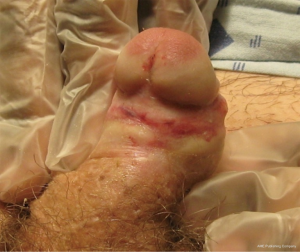
Distal urethral strictures confined to the fossa navicularis and meatus comprise approximately 18% of all anterior urethral strictures (2). These strictures can be idiopathic or occur as a result of instrumentation, trauma, prior hypospadias repair, or lichen sclerosus (3-8). Lichen sclerosus is the most commonly cited etiology of distal strictures in the literature, with rates of 12-42% (5-8). Lichen sclerosus is a chronic, often recurrent inflammatory dermatosis of unknown cause, which results in dermal thinning, scarring, and progressive fibrosis (Figure 1). It most commonly affects the glans and preputial skin and involves the urethra in 20-83% of cases in the literature (9-11). Urethral involvement can range from isolated meatal stenosis to obliterative stricture disease, which can encompass the entire anterior urethra. The cause of lichen sclerosus remains unknown, although infectious, autoimmune and genetic etiologies have been postulated and continue to be explored.
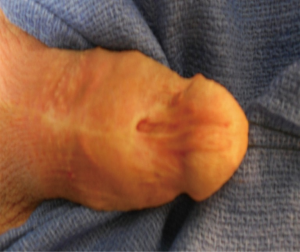
Despite refinements in the design of endoscopic instruments, cystourethroscopy for endoscopic procedures is among the leading iatrogenic causes of distal urethral strictures, with rates of 6.3-31% in the literature (5-7). Other common iatrogenic etiologies include prolonged urinary catheterization and complications of prior hypospadias repair (Figure 2) (5,12). While limited data specifically address the role of prior hypospadias repair in distal strictures, it has been described as a prevalent etiologic factor for urethral stricture disease. In a large multinational series of more than 2,500 men with urethral strictures, Stein et al. found that prior hypospadias repair was associated with 17% of strictures overall and 48% of iatrogenic strictures (4). Similarly in a single institutional series from Italy, Palminteri et al. reported that 12.2% of their strictures were attributable to prior surgery for hypospadias (3). Finally, prior ablative therapy for condyloma is a less frequent cause of distal strictures but is cited in several series on the management of distal urethral strictures and therefore deserves mention (5,13).
Diagnosis and evaluation
The goal of urethral stricture evaluation is to delineate the location, length, density and degree of underlying spongiofibrosis. Physical exam is perhaps the best, first diagnostic tool for distal strictures. Careful visual inspection of the meatus, glans and prepuce should reveal the hallmark fibrosis, retraction, and discoloration of lichen sclerosus, if present. Palpation of the glans and distal corpus spongiosum may define the extent of fibrosis. Retrograde urethrogram should be performed to delineate the urethral anatomy and exclude concomitant strictures involving the remainder of the anterior urethra. However, since the catheter utilized in retrograde urethrography may obscure much of the distal urethra, voiding urethrography can often provide superior radiographic assessment of the distal urethra. This can be performed either through retrograde instillation of diluted contrast media into the bladder or by intravenous administration of iodinated contrast (2 cc/kg) followed by fluoroscopy during voiding (12). Cystourethroscopy aids in the assessment of stricture density and allows visual survey of the urethra distal and proximal to the lesion. Use of a pediatric cystoscope or semi-rigid ureteroscope may allow for endoscopic visualization without dilation of the stricture if an adult cystoscope proves too large (14).
Principles of management
The notion that the urologist should proceed up the “reconstructive ladder”, exhausting endoscopic procedures and simple procedures before pursuing complex reconstruction has been refuted in the literature. This approach is often ineffective and does not limit patient morbidity, decrease disease progression, or minimize cost (15,16). Instead, a more prudent approach to the treatment of distal strictures is to determine which singular or staged intervention offers the patient the highest likelihood of durable patency with the least morbidity, while honoring patient-related goals. Ultimately, decision-making should be individualized, based on stricture burden, etiology, and patient motivation.
Dilation
The goal of dilation is to circumferentially disrupt the strictured tissue to restore the normal luminal diameter with minimal trauma. Thus in general, dilation is best suited for previously untreated, short strictures with minimal spongiofibrosis. However, the rate of recurrence following urethral dilation for strictures at all locations is as high as 85% at 2 years in the literature (17). There are no studies that specifically evaluate the efficacy of dilation for distal strictures. In the face of limited data, some have advocated that if urethral dilation is considered for management of distal urethral strictures, a periodic self-dilation protocol using soft catheters may be best (6,12). However, the efficacy of intermittent self-dilation for distal strictures is largely unstudied and may negatively affect quality of life. Lubahn et al. found that performance of intermittent self-dilation was associated with a perceived life interruption and poor overall quality of life in men with urethral strictures (18). Dilation should also be avoided in men with lichen sclerosus, as repeated instrumentation may exacerbate this inflammatory process and contribute to extension of disease. Given the dearth of evidence of long term efficacy and the potential for patient discomfort, strong consideration should be given to avoidance of dilation in favor of reconstructive approaches.
Meatotomy
Meatotomy can be utilized for the isolated meatal stricture with minimal or no fossa navicularis involvement. In simple meatotomy, the meatus is incised ventrally, and the urethral mucosal edges are re-approximated to glans skin using interrupted absorbable suture (5). Malone described a ‘plastic’ meatotomy for severe meatal stenosis related to lichen sclerosus (19). It differs from simple meatotomy in that dorsal and ventral meatal incisions are utilized to increase meatal caliber, while tissue mobilization and a counter-incision are used to preserve glans cosmesis. In this procedure a small ventral meatotomy is performed to allow the passage of forceps to evaluate for potential fossa navicularis involvement. Once fossa navicularis involvement greater than a few millimeters has been excluded, a deeper dorsal meatotomy into the glans is performed. The urethral mucosa is re-approximated to the glans skin with 5-0 to 7-0 absorbable suture. A relaxing, inverted V incision is made above the dorsal apex of the meatotomy, the skin edges are mobilized sharply, and the left and right inner edges are opposed with running 7-0 suture. The outer glans skin is closed with interrupted 6-0 or 7-0 absorbable suture (20). In the properly selected patient, simple and plastic meatotomy have excellent results, with reported success rates exceeding 80% at intermediate term follow-up (5,19).
Extended meatotomy
Extended meatotomy is considered separately because it is a useful tool for patients with meatal strictures with fossa navicularis involvement, who have either failed prior flap or graft reconstructive procedures or are reluctant for such reconstructive procedures. This procedure is performed by sharply incising the urethra in the ventral midline until the proximal lumen easily calibrates to 24-French and the urethral mucosa and glans tissue are healthy and supple. Fibrotic glans and indurated skin edges are excised and urethral mucosa is approximated to viable ventrolateral skin with interrupted 4-0 or 5-0 absorbable sutures. Extended meatotomy is technically straightforward, well tolerated, and can preserve a man’s ability to stand and void. Morey et al. report a success rate of 88% at 38 months follow-up with this approach (21). Of the two men who failed in this series, one was salvaged with a revision/repeat extended meatotomy and the other by perineal urethrostomy. The potential drawbacks of this procedure are compromised cosmesis of the glans with a hypospadiac meatus and spraying of the urinary stream secondary to the proximal position of the neomeatus. If patients are well counseled regarding expected outcomes, a high level of patient satisfaction can be achieved with this procedure.
Distal urethroplasty
Beyond simple and extended meatotomy, there are a multitude of open reconstructive techniques for distal urethral strictures in the reconstructive urologists’ armamentarium. Open distal urethroplasty is typically best reserved for the more extensive distal strictures involving the meatus, fossa navicularis, and pendulous urethra or for obliterative or recurrent strictures. The two most commonly used approaches include substitution grafting and flap urethroplasty. Within these approaches, there exist several variations in technique, including utilization of one or two-staged surgeries.
Flap urethroplasty
Flap urethroplasty for distal urethral strictures has been utilized for over a half century. The original reports of local penile flaps describe tubularized flaps or advancement flaps, plagued by complications, such as flap contraction, flap necrosis, penile torsion, or meatal tethering. The introduction of the fasciocutaneous ventral transverse island skin flap by Jordan in 1987 provided a reliable, easily producible technique for urologists (22). In this technique, a distal penile skin flap on a well-vascularized dartos fascial pedicle is mobilized and sutured to the urethra ventrally. Glans wings are created and brought over the flap for meatal/fossa navicularis strictures. Long-term success rates following this technique were excellent, with a stricture free rate of 83% among 35 patients at a mean follow up of 10.3 years (7). Others have adapted and slightly modified this technique with similar results. In 1998, Armenakas et al. described a technique of utilizing the ventral transverse skin flap while preserving the glans and elevating it off of the urethra (13). Armenakas et al. noted a success rate of 94% in their series of 18 patients at a mean follow up of 43 months. Later, Fiala et al. noted a 100% success rate at 35 months among 21 patients using the original flap technique described by Jordan (23). The authors also attempted to evaluate the cosmetic appearance of the reconstructed glans/penis, noting that all patients were “satisfied” with the cosmetic result. Flap urethroplasty techniques have also been applied to complex, long urethral strictures involving the pendulous urethra extending from the fossa navicularis proximally. McAninch and Morey described a technique using a circumferential penile circular fasciocutaneous flap for strictures up to 12-15 cm in length (24). They reported initial success rates of 79% for their cohort, with all recurrences being relatively short and occurring at either the distal or proximal anastomotic site. These results proved to be durable, as Whitson et al. later published a long-term success rate of 79% for distal penile circular fasciocutaneous flaps in 124 patients with a mean stricture length of 8 cm and mean follow up of 7.3 years (8). Morey et al. have asserted that total length of disease in fossa navicularis strictures is a predictor for recurrence (21). The authors stratified their fossa navicularis stricture patients into those with short (<2.5 cm) and long (>2.5 cm) strictures and found a significant difference in recurrence in the longer stricture group. Intuitively, the presence of lichen sclerosus is a contraindication to the use of the local penile skin flaps. Utilization of affected genital skin has proven to cause an increased risk of disease recurrence at the site of the flap. In the long-term study by the Virasoro group, all failures occurred in patients with a diagnosis of lichen sclerosus with an overall failure rate of 50% in this cohort of patients (7).
Substitution grafting
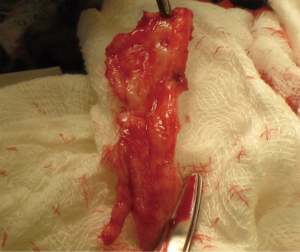
The use of grafting for distal urethral strictures is considered more versatile than flap urethroplasty and can be applied to strictures with inflammatory etiologies such as lichen sclerosus. In 1986, Devine described an initial experience with graft urethroplasty for distal urethral strictures involving the fossa navicularis using full-thickness penile skin (25). Since then, the use of grafts has evolved to include extragenital skin or mucosa, such as abdominal wall skin, postauricular skin, and bladder, intestinal, buccal or lingual mucosa (6). A key concept in the use of substitution grafts in distal urethral strictures, particularly for those due to lichen sclerosus, is excision of diseased tissue to create a healthy graft bed (Figure 3). Often this requires a two-stage procedure to ensure good graft take and successful tubularization. Venn and Mundy published their experience with distal urethral strictures in lichen sclerosus patients and compared a one-stage flap urethroplasty to two-stage grafting using non-genital skin (26). In the one-stage flap urethroplasty patients, 100% of patients had recurrence of disease, while only 6% of two-stage, nongenital skin graft patients failed. Depasquale et al. reported a 90% long-term recurrence rate in patients with lichen sclerosus, undergoing two-stage substitution grafting with genital skin compared to no recurrences in patients who underwent similar reconstruction with oral muscosa (9). For hypospadias related stricture disease, favorable outcomes have also been reported with a staged approach, particularly with buccal mucosa grafts (Figure 4). Meeks et al. reported an 86% success rate at 22 month follow up for patients who underwent staged urethroplasty after failure of prior hypospadias repair (20). The authors utilized a variety of graft types, including buccal mucosa (71%), hairless abdominal wall skin (14%), penile skin (7%) and post-auricular Barbagli (7%). Barbagli et al. examined their cohort of 60 adult hypospadias failure patients and determined an overall success rate of 67.7% for multi-staged procedures, with 82.3% success using buccal mucosa grafts and 50% success with penile skin grafts at 34 months (27). In a separate study of hypospadias-related complications, Barbagli et al. found that hypospadias patients require a median of five procedures before definitive treatment success was obtained (28). Both lichen sclerosus and hypospadias-related distal urethral strictures present challenging problems for reconstructive urologists and may require multi-staged interventions. Patient selection for surgical approach is crucial in both these populations, with the most durable results occurring after buccal mucosa grafting.
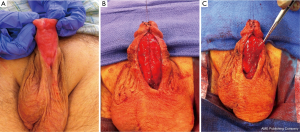
More recently, several institutions have applied one-stage onlay buccal mucosa grafting to distal urethral strictures including the fossa navicularis because often the urethral plate is salvageable in these strictures and the diseased segment does not require complete excision. Dubey et al. compared buccal mucosa dorsal onlay grafting to penile skin flap urethroplasty in a randomized controlled study for anterior urethral strictures involving the penile and bulbar urethra (29). The authors found similar success rates between the two groups (89.9% for buccal vs. 85.6% for penile flap) but noted patient satisfaction was significantly higher for the buccal mucosa group (89% vs. 65% for the penile flap, P=0.001), which the authors contended was related to penile skin flap complications (tethering, numbness, poor wound healing). Similarly, Barbagli et al. compared their outcomes with penile skin flap, one-stage onlay skin graft, and one-stage onlay oral mucosa graft for pendulous urethral strictures with success rates of 67%, 78%, and 82% respectively (28). In the study the grafts were applied in both a ventral and dorsal onlay fashion, with equivalent results. Dubey et al. also compared their experience with one-stage dorsal onlay to two-stage buccal mucosa grafting in lichen sclerosus related distal urethral strictures (30). At a follow-up of 33 months, 12% of one-stage onlay vs. 28% of two-stage patients developed recurrence, suggesting a favorable outcome for a one-stage approach in a suitably selected patient. Several other studies confirm these success rates for one-stage onlay buccal mucosa graft urethroplasty (11,31), which has quickly become the widely accepted graft of choice. In situations where buccal mucosa is not viable, several other mucosal graft choices exist, such as bladder, colonic, and lingual. Of these options, perhaps the most easily accessible and least morbid is the lingual mucosa graft. While no studies exist specifically examining its use in distal urethral strictures, several recent publications suggest comparable outcomes to buccal mucosa with short-term success rates ranging from 80-90% (32,33). An alternative to the one-stage mucosal onlay technique is the one-stage combination graft/flap method. Gelman and Sohn reported 100% success at a mean of 39 months in their series of 12 patients who underwent combined one-stage dorsal buccal mucosa graft and ventral penile skin flap onlay for obliterative distal urethral strictures (34). For patients with lichen sclerosus involvement, Goel et al. describe a double mucosa graft technique whereby the obliterated meatus/distal urethra is entirely replaced by a dorsal inlay buccal mucosa graft quilted to the glans along with a ventral onlay buccal mucosa graft (35). At a short-term follow-up of 13.5 months, the authors reported 100% success rate of the double buccal mucosa urethroplasty in 12 patients. While the abovementioned studies are limited in their sample sizes and exhibit variability in the selection, measurement, and definition of successful outcomes, there is a growing body of literature that supports open reconstruction of distal urethral strictures involving both the fossa navicularis and meatus as the gold standard treatment. Both flap urethroplasty and substitution grafting are reproducible and reliable in the hands of experienced reconstructive urologists with proper patient selection.
Conclusions
Distal urethral strictures involving the fossa navicularis and meatus present some of the most complex reconstructive challenges to urologists. In addition to maintaining anatomic patency of the urethra following repair, cosmesis is an important consideration. For short meatal strictures, meatotomy can be effective and durable. In more extensive strictures or for those due to lichen sclerosus, endoscopic or conservative approaches are generally unsuccessful. Open reconstruction of these distal strictures is the gold standard. With advancements in local flap and substitution grafting techniques, distal urethral strictures can now be effectively treated with excellent long-term success rates in properly selected patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667-74. [PubMed]
- Fenton AS, Morey AF, Aviles R, et al. Anterior urethral strictures: etiology and characteristics. Urology 2005;65:1055-8. [PubMed]
- Palminteri E, Berdondini E, Verze P, et al. Contemporary urethral stricture characteristics in the developed world. Urology 2013;81:191-6. [PubMed]
- Stein DM, Thum DJ, Barbagli G, et al. A geographic analysis of male urethral stricture aetiology and location. BJU Int 2013;112:830-4. [PubMed]
- Meeks JJ, Barbagli G, Mehdiratta N, et al. Distal urethroplasty for isolated fossa navicularis and meatal strictures. BJU Int 2012;109:616-9. [PubMed]
- Tonkin JB, Jordan GH. Management of distal anterior urethral strictures. Nat Rev Urol 2009;6:533-8. [PubMed]
- Virasoro R, Eltahawy EA, Jordan GH. Long-term follow-up for reconstruction of strictures of the fossa navicularis with a single technique. BJU Int 2007;100:1143-5. [PubMed]
- Whitson JM, McAninch JW, Elliott SP, et al. Long-term efficacy of distal penile circular fasciocutaneous flaps for single stage reconstruction of complex anterior urethral stricture disease. J Urol 2008;179:2259-64. [PubMed]
- Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int 2000;86:459-65. [PubMed]
- Dubey D, Kumar A, Mandhani A, et al. Buccal mucosal urethroplasty: a versatile technique for all urethral segments. BJU Int 2005;95:625-9. [PubMed]
- Kulkarni S, Barbagli G, Sansalone S, et al. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int 2009;104:1150-5. [PubMed]
- Armenakas NA, McAninch JW. Management of fossa navicularis strictures. Urol Clin North Am 2002;29:477-84. [PubMed]
- Armenakas NA, Morey AF, McAninch JW. Reconstruction of resistant strictures of the fossa navicularis and meatus. J Urol 1998;160:359-63. [PubMed]
- Figueroa JC, Hoenig DM. Use of 7.5F flexible pediatric cystoscope in the staging and management of urethral stricture disease. J Endourol 2004;18:119-21. [PubMed]
- Hampson LA, McAninch JW, Breyer BN. Male urethral strictures and their management. Nat Rev Urol 2014;11:43-50. [PubMed]
- Greenwell TJ, Castle C, Andrich DE, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol 2004;172:275-7. [PubMed]
- Veeratterapillay R, Pickard RS. Long-term effect of urethral dilatation and internal urethrotomy for urethral strictures. Curr Opin Urol 2012;22:467-73. [PubMed]
- Lubahn JD, Zhao LC, Scott JF, et al. Poor quality of life in patients with urethral stricture treated with intermittent self-dilation. J Urol 2014;191:143-7. [PubMed]
- Malone P. A new technique for meatal stenosis in patients with lichen sclerosus. J Urol 2004;172:949-52. [PubMed]
- Steffens JA, Anheuser P, Treiyer AE, et al. Plastic meatotomy for pure meatal stenosis in patients with lichen sclerosus. BJU Int 2010;105:568-72. [PubMed]
- Morey AF, Lin HC, DeRosa CA, et al. Fossa navicularis reconstruction: impact of stricture length on outcomes and assessment of extended meatotomy (first stage Johanson) maneuver. J Urol 2007;177:184-7. [PubMed]
- Jordan GH. Reconstruction of the fossa navicularis. J Urol 1987;138:102-4. [PubMed]
- Fiala R, Vrtal R, Zenisek J, et al. Ventral prepucial flap meatoplasty in the treatment of distal urethral male strictures. Eur Urol 2003;43:686-8. [PubMed]
- McAninch JW, Morey AF. Penile circular fasciocutaneous skin flap in 1-stage reconstruction of complex anterior urethral strictures. J Urol 1998;159:1209-13. [PubMed]
- Devine CJ Jr. Surgery of the Urethra. In: Walsh PCea. eds. Campbell’s Urology, 5th Edition. Philadelphia: W.B. Saunders;1986:2853-85.
- Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol 1998;81:735-7. [PubMed]
- Barbagli G, De Angelis M, Palminteri E, et al. Failed hypospadias repair presenting in adults. Eur Urol 2006, 49:887-94; discussion 895. [PubMed]
- Barbagli G, Morgia G, Lazzeri M. Retrospective outcome analysis of one-stage penile urethroplasty using a flap or graft in a homogeneous series of patients. BJU Int 2008;102:853-60. [PubMed]
- Dubey D, Vijjan V, Kapoor R, et al. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol 2007;178:2466-9. [PubMed]
- Dubey D, Sehgal A, Srivastava A, et al. Buccal mucosal urethroplasty for balanitis xerotica obliterans related urethral strictures: the outcome of 1 and 2-stage techniques. J Urol 2005;173:463-6. [PubMed]
- Singh BP, Pathak HR, Andankar MG. Dorsolateral onlay urethroplasty for anterior urethral strictures by a unilateral urethral mobilization approach. Indian J Urol 2009;25:211-4. [PubMed]
- Singh PB, Das SK, Kumar A, et al. Dorsal onlay lingual mucosal graft urethroplasty: Comparison of two techniques. Int J Urol 2008;15:1002-5. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. The use of lingual mucosal graft in adult anterior urethroplasty: surgical steps and short-term outcome. Eur Urol 2008;54:671-6. [PubMed]
- Gelman J, Sohn W. 1-stage repair of obliterative distal urethral strictures with buccal graft urethral plate reconstruction and simultaneous onlay penile skin flap. J Urol 2011;186:935-8. [PubMed]
- Goel A, Dalela D, Sankhwar SN. Meatoplasty using double buccal mucosal graft technique. Int Urol Nephrol 2009;41:885-7. [PubMed]