Advances in ureteral stents
Introduction
Ureteric stent placement is one of the most common urological procedures. The elusive “perfect stent” has yet to be found. Ideal stent characteristics include ease of placement and removal, lack of upper tract and lower tract irritative voiding symptoms, maintenance of excellent urine flow to optimize upper tract drainage, resistance to infection and encrustation, and degradability if forgotten. Current indications for stent placement include relief of ureteral obstruction whether the cause is intrinsic (from a calculus, clot, or urothelial carcinoma) or extrinsic (from external compression or mass effect such as lymphadenopathy). Other indications include maintenance of ureteral patency for healing after ureteral and upper tract reconstruction, endoscopy or trauma. Ureteric stents have been shown to be associated with morbidity in up to 80% of patients with the most common side effects being flank pain, hematuria, irritative voiding symptoms, and reduced work capacity (1,2). We are currently in an era of exciting and innovative technological advancements in stent material, properties, coatings, and design, drawing from the expertise of urological surgeon scientists, biomedical engineers, and microbiologists. We will review a variety of new advancements in stent technology, the theories behind their development and implementation, and the current evidence for their clinical use.
Infection
Bladder infection is associated with ureteral stents in approximately 20-45% of cases and is dependent on the duration of stenting (3-5). Within a matter of hours of ureteral stent placement, a conditioning film forms and extra polysaccharide matrix and “slime” deposit on the surface which then engulf bacteria which makes it very difficult to eradicate the bacteria for three reasons: (I) the antibiotics cannot penetrate the biofilm; (II) the bacteria enter into a suspended metabolic state which are not susceptible to antibiotics that typically attack rapidly metabolizing bacteria; and (III) resistance genes are upregulated rendering the antibiotics ineffective (6).
Gel-based stent
Rosman et al. describe a novel stent that is gel-based and composed of hydrated, partially hydrolyzed polyacrylonitrile [pAguaMedicinaTM Pediatric Ureteral Stent (pAMS), Q Urological, Natick, MA] (7). They assessed it for bacterial biofilm and compared it against a typical plastic (ethylene vinyl acetate) stent and found that their novel stent decreased bacterial adherence up to 70% in an in vitro environment. Biofilm formation was still present on the pAMS stent, but the time to accumulation was prolonged compared to the control stent leading the authors to speculate that this may reduce the chances of stent-related infection. We will await to see human trials of this novel stent. There is no mention if this stent is only for use in pediatrics or if it will be used in adults as well. Lastly, it will be interesting to see how patient comfort and removal of this novel material will be since it is gel-based, and presumably a softer material than plastic.
Combining antibiotics to work synergistically is a concept widely used clinically as in the example of Pseudomonas or Tuberculosis. Minardi et al. examined the combination of two antibiotics, rifampin and tigecycline, both in in vitro and in vivo infection models. Ureteral stents were soaked in rifampin for coating and tigecycline was injected intraperitoneally into a rat model infected with Enterococcus (8). The combination of the two antibiotics was more effective against bacteria than either of the antibiotics alone, although the single antibiotics were also effective as well. No other bacteria were tested besides Enterococcus and this proof-of-principle study showed that the combination of antibiotics was effective. One could question the systemic delivery (intraperitoneal injection) of tigecycline versus stent elution alone and the fact that only Enterococcus was tested compared to other bacteria that can also adhere to ureteral stents. It would be nice to see both antibiotics eluted from the stent, if possible, as well as testing against additional bacteria such as E. coli. The same group has also tested the combination of clarithromycin and amikacin against Pseudomonas (9). It will be interesting to see clinical data in the future and if this will help stent-related infection without giving systemic antibiotics.
Slow-release varnish coating
An Israeli group evaluated C-flex material (Cook Medical, Bloomington, IN) coated with a medical antiseptic inside of a slow release chlorhexidine varnish against Enterococcus, Escherichia, and Pseudomonas in an in vitro model (10). The active ingredient was chlorhexidine (1% or 2%) prepared in ethyl cellulose, polyethylene glycol, and KlucelTM EF. The chlorhexidine slow release varnish performed very effectively for all three bacteria tested and essentially prevented colonization. This method of varnish could be used to incorporate other antimicrobials onto the surface of the stent and the in vitro tests warrant future clinical evaluation. The release of antiseptic from the varnish seems to be stable and controlled as the stent was still clean at several time points.
Antimicrobial triclosan
Triclosan is a broad-spectrum antimicrobial that has widespread use in medicine (sutures) as well as in general use (plastics etc.). It was the first compound to be approved for clinical use in an eluting ureteral stent. In patients who received chronic long-term stenting, triclosan-eluting ureteral stents changed every 3 months did not result in reduced infection rates in this difficult patient population who are chronically colonized with bacteria (11). In a separate study, the rate of bacteriuria was the same in triclosan-eluting stents and controls; however, the rate of symptoms and requirement for antibiotics for treatment were significantly lower in the triclosan-eluting group (11). Importantly, no patients developed organisms that became resistant to antibiotics unlike those patients who received oral antibiotics who had a higher rate of developing antibiotic resistance. One theory of stent symptoms is that bacterial colonization causes edema and inflammation that result in more symptoms from the inflammatory cytokines that are elicited by the stent (12). Even though bacteria are still present on the triclosan stent, live-dead staining have shown many of those adherent bacteria to be dead; this may be an advantage for triclosan in reducing symptomatic infections (13). This stent is no longer used clinically due to fears that it may induce bacterial resistance although that claim has been scientifically unfounded. The quest to reduce stent infection continues and various methods may require incorporation to be an effective tool.
New stent designs: helical stent
The PercuflexTM Helical (Boston Scientific) stent is made of the PercuflexTM material and is modified with a spiral-cut along the entire length of the straight portion of the stent designed to conform to the shape of the ureter by increasing the flexibility of the ureteral portion without limiting flow (Figure 1). It was designed based on the theory that a stent that is better able to conform to the ureter, while providing adequate drainage, may reduce upper tract related symptoms associated with indwelling ureteric stents (14). The distal and proximal portions consist of a standard double-pigtail shape with standard stent material. Mucksavage et al. used an in vivo swine model to compare acute and chronic flow characteristics of the Helical stent versus a control ureteric (15). They compared flows in an unobstructed ureter, a stented ureter, an intraluminally-obstructed stent, and an extraluminally-obstructed stent. They also assessed performance and hydronephrosis on pyelograms, and histopathologic changes in animals who had the stent placed for 10 days. They found that the helical stent appeared to conform better to the shape of ureter in both acute and chronic animals and the helical stent drained as well as a standard stent. Acute and chronic flow characteristics in the helical stent were equivalent to the control stent. There was no difference in the degree of hydronephrosis, stent migration, and presence of urinary tract infection. As well, there was no difference in histopathologic grading or degree of encrustation between stents. This evidence is encouraging from a flow characteristics and histopathologic perspective for the helical stent. Human clinical trials are needed to discern whether the improved ureteric conformance with the helical stent may decrease stent-related symptoms.
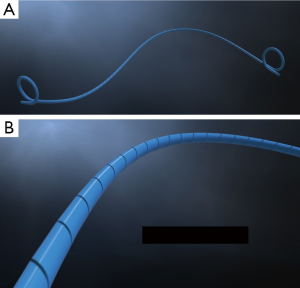
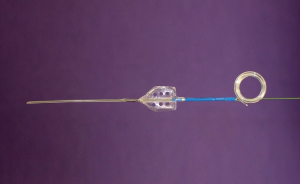
Guidewire-based MicrostentTM
In the setting of acute ureteral obstruction secondary to stone disease, it is advantageous for a ureteral stent to create maximal dilatation while occupying as little space as possible within the lumen of the ureter, to facilitate stone passage and relief of upper urinary tract obstruction. The Microstent (PercSys) is a 3 Fr stent that is based on a guidewire with a distal bladder anchoring coil and a novel proximal film anchoring mechanism that anchors in the ureter immediately proximal to a ureteral calculus (Figures 2,3). The potential risk of using a smaller stent is that it may not provide adequate drainage of the obstructed system. To determine if this smaller stent drained well, Lange et al. performed a study with an in vitro and ex vivo porcine model to measure mean flow rates through obstructed and unobstructed ureters with either a 3 Fr Double-J stent (Cook), 3 Fr Microstent (PercSys) or standard 4.7 Fr Double-J stent (Cook) (16). They found the 3 Fr Microstent demonstrated drainage equivalent to the standard 4.7 Fr Double-J stent, in both in vitro silicone and ex vivo porcine obstructed urinary models. Further studies are needed to examine whether there are improved rates of stone passage with the Microstent, given its minimal occupation of space within the ureter. A clinical trial is ongoing at our institution to evaluate the use of the Microstent in patients with acutely obstructing ureteral stones. This stent can be delivered similarly to a guidewire through a flexible ureteroscope directly into the ureter. Removal of the proximal portion of the Microstent inside the scope then deploys the proximal anchoring film inside the ureter and releases the stent. This novel stent may prove useful in relieving acute obstruction caused by ureteral stones.
Anti-refluxing stents
Placement of a double-J stent after kidney transplant is to protect the uretero-vesical anastamosis to reduce incidence of fistula and stenosis at the anastamosis. However, the double J stent can cause vesico-ureteric reflux (VUR), predisposing to graft infections which can be a significant source of morbidity for the graft. In non-transplanted kidneys, ureteral stents are known to cause reflux, particularly with voiding, that results in significant flank pain for patients. An antireflux stent could potentially eliminate this complication in both patient populations. A single-centre prospective randomized study of 44 kidney transplant recipients who were randomized to receive either the standard pediatric 4.8 Fr double J stent or the anti-reflux stent device after a Lich-Gregoire antireflux ureteroneocystostomy, with a ureteral tunnel at least 2 cm long (17). Results showed no statistically significant difference in either incidence of VUR and UTIs, or the short-term outcome of the grafts (at 12 months). It was concluded that the anti-reflux device did not have an impact on the incidence of stent-related side effects or complications. It would be interesting to determine if these stents result in decreased symptoms in a non-transplant, native kidney population.
Biodegradable ureteral stents
The forgotten stent is a complication feared by urologists, as it has been associated with morbidity, potential kidney loss, and even death (18,19). A biodegradable stent would eliminate these risks, as well as eliminate the need for the patient to undergo a stent removal procedure and the problems of chronic indwelling stents (encrustation, stone formation, infection) (20,21). In 2008, Hadaschik et al. performed an in vivo porcine model study comparing drainage, degree of hydronephrosis, ureteral dilatation, and urinary tract infection rates between a degradable L-glycolic acid (UripreneTM, Poly-Med Inc., Anderson, SC) stent (Figure 4) and a standard biostable control stent (22). The animals were sacrificed at 2, 4, 7, and 10 weeks to determine stent degradation. Results showed that the Uriprene stents began to degrade at 3 weeks and were completely degraded by 10 weeks. Uriprene stents provided similar drainage to regular stents, and the uriprene stents resulted in less ureteral dilatation and fewer positive urine cultures. However, the axial rigidity was too soft resulting in difficulty advancing the stent directly over a guidewire. As well, it was thought that 7-10 weeks was longer than stents are typically left indwelling.

A second generation was developed to degrade faster. Chew et al. performed a study of the next generation biodegradable uriprene ureteral stent in a Yucatan pig model (20,21). They hypothesized that these stents would degrade faster than 7-10 weeks and reinforced to provide better axial rigidity, thus easing the insertion over a regular polytetrafluoroethylene guidewire. Results were encouraging with 80% of stents degrading over 2-3 weeks with 100% of stents completely eliminated by week 4. The Uriprene stent was very biocompatible on histology with no hydronephrosis in comparison to control biostable stents. Drainage was also superior to control stents as seen by weekly intravenous pyelograms. We are currently embarking on a first-in-human clinical trial with this biodegradable stent at our institution.
Novel design and combination material: AlliumTM stent
A self-expanding stent was developed to provide minimally invasive treatment for congenital ureteropelvic junction obstruction and iatrogenic ureteral stenosis. This stent ranges in size from 24 to 30 Fr and consists of an expanding metal design that is covered by a thin polyurethane film that prevents tissue ingrowth into the stent (Allium stent, Allium Medical, Caesarea Industrial Park South, Israel). Insertion of the 30 Fr self-expanding Allium stent was used by Leonardo et al. to treat 12 adult patients from 2010-2013 with hydronephrosis due to either congenital UPJ obstruction or iatrogenic ureteral stenosis (23). Median follow up was 10 months after stent removal, and all patients were pain free immediately after the procedure, and were free of complications, with complete correction of the stenotic lesion. No evidence of recurrence of stenosis during follow-up. The stent is removable and is not designed to be permanent. Further studies in larger patient cohorts with long term follow up are needed, and the initial results are encouraging.
Conclusions
Thorough overviews of ureteral stents and polymers (24), metal ureteral stents (25-27), and infection related to ureteral stents (28) are reviewed elsewhere. Unfortunately, there is no panacea to solve the ills of stent-related problems such as infection, symptoms, and encrustation. New designs, coatings, and materials may help to reduce these symptoms.
Acknowledgements
Funding: This work was supported by the AUA Care Foundation Segura Scholarship (D. Lange), Michael Smith Foundation for Health Research (B. Chew), Vancouver Coastal Health Research Institute (B. Chew), Canadian Urology Association-Astellas Grant, and the Canadian Institute for Health Research.
Footnote
Conflicts of Interest: Drs. Chew and Lange are consultants with Bard Medical, Boston Scientific Corporation, Cook Medical, Olympus, Percutaneous Systems and Poly-Med Inc. The other author has no conflicts of interest to declare.
References
- Joshi HB, Stainthorpe A, MacDonagh RP, et al. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 2003;169:1065-9. [PubMed]
- Joshi HB, Okeke A, Newns N, et al. Characterization of urinary symptoms in patients with ureteral stents. Urology 2002;59:511-6. [PubMed]
- Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining Double-J catheter colonization. J Endourol 2009;23:1015-9. [PubMed]
- Rahman MA, Alam MM, Shamsuzzaman SM, et al. Evaluation of bacterial colonization and bacteriuria secondary to internal ureteral stent. Mymensingh Med J 2010;19:366-71. [PubMed]
- Ozgur BC, Ekıcı M, Yuceturk CN, et al. Bacterial colonization of double J stents and bacteriuria frequency. Kaohsiung J Med Sci 2013;29:658-61. [PubMed]
- Denstedt JD, Cadieux PA. Eliminating biofilm from ureteral stents: the Holy Grail. Curr Opin Urol 2009;19:205-10. [PubMed]
- Rosman BM, Barbosa JA, Passerotti CP, et al. Evaluation of a novel gel-based ureteral stent with biofilm-resistant characteristics. Int Urol Nephrol 2014;46:1053-8. [PubMed]
- Minardi D, Cirioni O, Ghiselli R, et al. Efficacy of tigecycline and rifampin alone and in combination against Enterococcus faecalis biofilm infection in a rat model of ureteral stent. J Surg Res 2012;176:1-6. [PubMed]
- Cirioni O, Ghiselli R, Silvestri C, et al. Effect of the combination of clarithromycin and amikacin on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. J Antimicrob Chemother 2011;66:1318-23. [PubMed]
- Zelichenko G, Steinberg D, Lorber G, et al. Prevention of initial biofilm formation on ureteral stents using a sustained releasing varnish containing chlorhexidine: in vitro study. J Endourol 2013;27:333-7. [PubMed]
- Cadieux PA, Chew BH, Nott L, et al. Use of triclosan-eluting ureteral stents in patients with long-term stents. J Endourol 2009;23:1187-94. [PubMed]
- Elwood CN, Lange D, Nadeau R, et al. Novel in vitro model for studying ureteric stent-induced cell injury. BJU Int 2010;105:1318-23. [PubMed]
- Chew BH, Cadieux PA, Reid G, et al. In-vitro activity of triclosan-eluting ureteral stents against common bacterial uropathogens. J Endourol 2006;20:949-58. [PubMed]
- Chew BH, Knudsen BE, Nott L, et al. Pilot study of ureteral movement in stented patients: first step in understanding dynamic ureteral anatomy to improve stent comfort. J Endourol 2007;21:1069-75. [PubMed]
- Mucksavage P, Pick D, Haydel D, et al. An in vivo evaluation of a novel spiral cut flexible ureteral stent. Urology 2012;79:733-7. [PubMed]
- Lange D, Hoag NA, Poh BK, et al. Drainage characteristics of the 3F MicroStent using a novel film occlusion anchoring mechanism. J Endourol 2011;25:1051-6. [PubMed]
- Battaglia M, Ditonno P, Selvaggio O, et al. Double J stent with antireflux device in the prevention of short-term urological complications after cadaveric kidney transplantation: single-center prospective randomized study. Transplant Proc 2005;37:2525-6. [PubMed]
- Bogdanovic J, Djozic J. Images in clinical medicine. Stent-associated urinary calculi. N Engl J Med 2009;361:612. [PubMed]
- Dahl DM, Mueller PR, Young RH. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 34-2004. A 45-year-old man with recurrent pain in the right flank and hematuria. N Engl J Med 2004;351:2102-10. [PubMed]
- Chew BH, Paterson RF, Clinkscales KW, et al. In vivo evaluation of the third generation biodegradable stent: a novel approach to avoiding the forgotten stent syndrome. J Urol 2013;189:719-25. [PubMed]
- Chew BH, Lange D, Paterson RF, et al. Next generation biodegradable ureteral stent in a yucatan pig model. J Urol 2010;183:765-71. [PubMed]
- Hadaschik BA, Paterson RF, Fazli L, et al. Investigation of a novel degradable ureteral stent in a porcine model. J Urol 2008;180:1161-6. [PubMed]
- Leonardo C, Salvitti M, Franco G, et al. Allium stent for treatment of ureteral stenosis. Minerva Urol Nefrol 2013;65:277-83. [PubMed]
- Venkatesan N, Shroff S, Jayachandran K, et al. Polymers as ureteral stents. J Endourol 2010;24:191-8. [PubMed]
- Kallidonis PS, Georgiopoulos IS, Kyriazis ID, et al. Drug-eluting metallic stents in urology. Indian J Urol 2014;30:8-12. [PubMed]
- Kulkarni R. Metallic stents in the management of ureteric strictures. Indian J Urol 2014;30:65-72. [PubMed]
- Chow PM, Hsu JS, Huang CY, et al. Metallic ureteral stents in malignant ureteral obstruction: clinical factors predicting stent failure. J Endourol 2014;28:729-34. [PubMed]
- Chew BH, Lange D. Ureteral stent symptoms and associated infections: a biomaterials perspective. Nat Rev Urol 2009;6:440-8. [PubMed]