What lessons can be learned from testicular histology in undescended testes?
Introduction
A considerable amount of information has been discovered from thousands of testicular biopsies that have been performed in young boys at the time of orchidopexy. In this review we highlight important findings that have changed or helped to guide our clinical decision making in the treatment of boys with cryptorchidism. Initially it was thought that the most clinically significant findings in testicular biopsies were the total number or concentration of germ cells, the degree of fibrosis, and presence of intratubular germ cell neoplasia (ITGCN) or carcinoma in situ. Later studies further defined the effects of cryptorchidism on the normal develop of the germ cells and Leydig cells. The presence or absence of the normal development and transformation of fetal germ cells or gonocytes into adult stem cells or adult dark (Ad) spermatogonia is the single most important histological predictor of future functional status of cryptorchid testes. It is now understood that certain immunohistochemical markers initially used to detect ITGCN also detect fetal gonocytes and can be used to determine defects in development of the germ cells in cryptorchid testes. Currently, the important predictors of future impairment of testicular function in boys with cryptorchidism treated with orchidopexy are: the persistence of fetal gonocytes after 6 months of age, absence or decrease in the number of Ad spermatogonia, and absence of primary spermatocytes by 3-5 years of age. When taken into consideration with certain key clinical factors (history of bilateral cryptorchidism, hormonal status, and age at orchidopexy) these histological findings are important in determining the follow up and need for additional diagnostic testing in these boys.
Normal development of leydig cells and male germinal epithelium
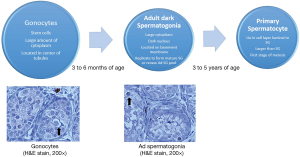
There are two major steps in maturation that occur in the developing tests prior to puberty. The first step normally occurs between 2-3 months of age and is associated with the transformation of gonocytes (fetal stem cells) into the Ad spermatogonia (adult stem cells) that will provide a self-replicating pool of cells that will fuel future spermatogenesis (1,2). This is associated with a marked reduction in the total number of germ cells per tubule and is usually completed by 6 months of age. This initial stage corresponds with a surge of luteinizing hormone and follicular stimulating hormone (3,4). Fetal Leydig cells proliferate and release a surge of testosterone in response to the rise in gonadotropins. The second step of the maturation process is associated with the transformation of germ cells to primary spermatocytes (first stage of meiosis) which occurs at 3-5 years of age (1). Figure 1 depicts the normal development of the male germinal epithelium.
Testicular maldescent and leydig cell function and development
A blunted response to the normal postnatal LH and FSH surge has been reported in cryptorchid testes. This is associated with a reduction in the postnatal rise in testosterone levels in cryptorchid boys (5). Hadziselimović et al. found that boys with cryptorchid testis (29 infants) had a significantly lower concentration of Leydig cells compared to controls testes (34 patients) (1). These findings were later confirmed by Huff et al. who examined 37 testicular biopsies from undescended testes in boys with unilateral cryptorchidism and compared them to 40 controls (biopsies at time of herniorraphy, hydrocelectomy, and autopsies). Hypoplasia of Leydig cells was evident in the first 2 months of life and the normal increase in proliferation of Leydig cells at 3-4 months did not occur in the cryptorchid tests (2). A decrease in the number of germ cells per tubule and failure of the development of Ad spermatogonia in cryptorchid testes became obvious by 4 months of life. Tasian et al. also examined biopsies from 274 older boys who underwent orchidopexy (6). They showed that Leydig cell function is important in the transformation of gonocytes to Ad spermatoginia. Zivkovic et al. examined testis biopsies from children at the time of unilateral orchidopexy with and without human chorionic gonadotropin stimulation (7). Hormonally treated boys had larger numbers of Ad spermatogonia. They also found that boys with normal Ad spermatogonia per tubule ratios had significantly higher total testosterone levels. Leydig cell dysfunction correlates negatively with age at orchidopexy and has deleterious effects on germ cell maturation in cryptorchid testes.
Testicular maldescent and germ cell development in the undescended testis and its contralateral partner
The total germ cell count decreases significantly the older a boy is at the age of orchidopexy (6,8-11). An increase in fibrosis of the cryptorchid testis parallels a loss in the total number of germ cells and is also more pronounced the older a child is at the time of orchidopexy (12,13). A decrease in the total number of germ cells is detectible just before 2 years of age (10,11). However, the effects of cryptorchidism on the maturation of spermatogonia are seen much earlier. The transformation of gonocytes to Ad spermatogonia that normally occurs at three months of age is delayed in cryptorchid boys (1,10,11). The disappearance of gonocytes is delayed up to 12 months in cryptorchid testes and up to 8 months in the contralateral descended testes (11). Cryptorchidism leads to a decrease in the total number of germ cells and the maturation of spermatogonia after 1 year of age (9,11). Although affected to a lesser degree, contralateral partners of undescended testes show signs of delayed maturation with decreased Ad spermatogonia and in the development primary spermatocytes (11).
Many early studies in undescended testes reported the total germ cell concentrations in undescended testes but did not further characterize the germinal epithelium. Huff et al. measured five parameters: total germ cells, gonocytes, adult spermatogonia, primary spermatocytes, and Leydig cell concentrations in 459 cryptorchid testes and 356 of their descended partners (9). The mean number of germ cells was not significantly different between contralateral descended and cryptorchid testes before 12 months of age. After 1 year the total number of germ cells in the cryptorchid testes approached zero whereas the concentration of germ cells remained stable in descended contralateral testes. Gonocytes were the only cell type that was higher in concentration in the cryptorchid testes between 6 and 18 months of age. Their concentration then dropped to zero after 18 months. Three abnormalities were reported in Ad spermatogonia: they appeared later in cryptorchid testes, the mean number of Ad spermatogonia decreased in first months of life and with the age at orchidopexy, and they appeared in fewer cryptorchids than descended testes. Primary spermatocytes did not appear in descended testes until 3 years of age and were found in only 2 out of 356 cryptorchid testes. This demonstrated that maturation of spermatogonia fails in cryptorchid testes and may result in a loss in the total number of germ cells in adulthood.
Cryptorchid testes and fertility potential
Proposed histologic markers for future fertility include the concentration of Ad spermatogonia per tubule ratio (Ad/T), the number of placental alkaline phosphatase (PLAP) positive cells (marker for gonocytes and undifferentiated germ cells), and total germ cell numbers. There is no doubt that boys who undergo orchidopexy at and older age (over 2 years) or who have bilateral cryptorchidism are at significant risks for infertility. Thorup et al. studied 89 boys (median age 1.8 years) who underwent bilateral orchidopexy and testicular biopsy and compared their histology to gonadotropin and inhibin B levels (14). They also quantified the number of gonocytes (positive cells) per testis. PLAP is found in gonocytes and primitive germ cells. It has been used as a marker for both fetal gonocytes and ITGCN. There was a significant positive correlation with the number of Ad spermatogonia and gonocytes and fertility potential (defined as normal total germ cell concentration and normal inhibin B and gonadotropin levels). Another study that followed up 70 patients with bilateral orchidopexy examined 35 patients with records of a fertility evaluation. The total number of germ cells per tubule and the age at orchidopexy significantly correlated with fertility. Fifty five percent of these men had and abnormal semen analysis. All men with a history of bilateral undescended testes are at significant risks of infertility.
In a study of 25 patients (mean age at orchidopexy was 9 years) with follow up after 18 years of age, Rusnack et al. found that boys with severe histology (marked decrease in total germ cell counts) were more likely to have a sperm densities of less than 20 million/cc (57%) compared to those with moderate histology (0%) (15). Those with bilateral undescended testes had uniformly poor semen analysis (11 boys) (78%) irrespective of testicular pathology. Fertility potential was assessed in 140 boys in a study of 1,335 cryptorchid boys that had a testicular biopsy at the time of orchidopexy. The number of spermatogonia per tubule correlated with the semen analyses in boys with a history of bilateral but not unilateral cryptorchidism (16). Risk for infertility was 78-100% (depending on testis biopsied) with a history of bilateral vs. 33% of unilateral cryptorchidism if the testicular biopsy of the undescended testis showed no presence of germ cells. The youngest patient without germ cells in the biopsy was 18 months. This argues that the total number of germ cells per tubule is not a good independent predictor of infertility in boys with unilateral cryptorchidism.
A more reliable histologic indicator of future fertility concerns in boys with unilateral cryptorchidism is the Ad spermatogonia per tubule ratio. Hadziselimovic and Herzog analyzed semen analyses from 21 unilateral and 6 bilateral cryptorchid patients who had an orchidopexy before 2 years of age. All boys who were younger than 6 months at surgery had a normal number of germ cells per tubule and those older than 6 months were all abnormal (17). However, 36% of those less than 6 months of age’s boys still had abnormal semen analyses despite successful orchidopexy. Those males who had Ad spermatogonia present at orchidopexy had a normal semen analysis 20 years later. Kraft et al. examined 85 patients who had a semen analyses after age 18 and a history of unilateral orchidopexy (mean age was 7.8 years). They found that mean sperm count and density was significantly decreased in the group with abnormal Ad/T concentrations (18). It is important to note that the sperm counts and densities, although significantly lower than normal, still remained in the normal range. The majority of men with a history of unilateral cryptorchidism who undergo orchidopexy have normal fertility potential.
Conclusions
Histological studies of testicular biopsies have helped to unravel the pathophysiology of cryptorchidism and contributed to the foundation of the clinical management of cryptorchidism. Several key lessons have been learned from past histological studies. Orchidopexy should be performed at an early age (6 months to 1 year) to prevent a permanent loss of functional germ cells. Biopsy specimens from cryptorchid testes should be analyzed for total germ cell concentration, presence of Ad spermatogonia, Ad spermatogonia/tubule ratio, presence of gonocytes (PLAP positive cells), presence of primary spermatocytes (in boys older than 3 years of age), and the degree of fibrosis. These findings have significant prognostic value regarding future fertility potential in boys with a history of orchidopexy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hadziselimović F, Thommen L, Girard J, et al. The significance of postnatal gonadotropin surge for testicular development in normal and cryptorchid testes. J Urol 1986;136:274-6. [PubMed]
- Huff DS, Hadziselimović F, Snyder HM 3rd, et al. Early postnatal testicular maldevelopment in cryptorchidism. J Urol 1991;146:624-6. [PubMed]
- Winter JS, Hughes IA, Reyes FI, et al. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J Clin Endocrinol Metab 1976;42:679-86. [PubMed]
- Forest MG, Sizonenko PC, Cathiard AM, et al. Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy. J Clin Invest 1974;53:819-28. [PubMed]
- Job JC, Toublanc JE, Chaussain JL, et al. The pituitary-gonadal axis in cryptorchid infants and children. Eur J Pediatr 1987;146:S2-5. [PubMed]
- Tasian GE, Hittelman AB, Kim GE, et al. Age at orchiopexy and testis palpability predict germ and Leydig cell loss: clinical predictors of adverse histological features of cryptorchidism. J Urol 2009;182:704-9. [PubMed]
- Zivkovic D, Bica DT, Hadziselimovic F. Relationship between adult dark spermatogonia and secretory capacity of Leydig cells in cryptorchidism. BJU Int 2007;100:1147-9. [PubMed]
- Suskind A, Hayner-Buchan A, Feustel PJ, et al. Fibrosis correlates with detailed histological analysis of human undescended testes. BJU Int 2008;101:1441-5. [PubMed]
- Huff DS, Hadziselimovic F, Snyder HM 3rd, et al. Histologic maldevelopment of unilaterally cryptorchid testes and their descended partners. Eur J Pediatr 1993;152:S11-4. [PubMed]
- Huff DS, Hadziselimovic F, Snyder HM 3rd, et al. Postnatal testicular maldevelopment in unilateral cryptorchidism. J Urol 1989;142:546-8. [PubMed]
- Huff DS, Fenig DM, Canning DA, et al. Abnormal germ cell development in cryptorchidism. Horm Res 2001;55:11-7. [PubMed]
- Suskind A, Hayner-Buchan A, Feustel PJ, et al. Fibrosis correlates with detailed histological analysis of human undescended testes. BJU Int 2008;101:1441-5. [PubMed]
- Park KH, Lee JH, Han JJ, et al. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int J Urol 2007;14:616-21. [PubMed]
- Thorup J, Kvist K, Clasen-Linde E, et al. The relation between adult dark spermatogonia and other parameters of fertility potential in cryptorchid testes. J Urol 2013;190:1566-71. [PubMed]
- Rusnack SL, Wu HY, Huff DS, et al. Testis histopathology in boys with cryptorchidism correlates with future fertility potential. J Urol 2003;169:659-62. [PubMed]
- Cortes D, Thorup JM, Visfeldt J. Cryptorchidism: aspects of fertility and neoplasms. A study including data of 1,335 consecutive boys who underwent testicular biopsy simultaneously with surgery for cryptorchidism. Horm Res 2001;55:21-7. [PubMed]
- Hadziselimovic F, Herzog B. Importance of early postnatal germ cell maturation for fertility of cryptorchid males. Horm Res 2001;55:6-10. [PubMed]
- Kraft KH, Canning DA, Snyder HM 3rd, et al. Undescended testis histology correlation with adult hormone levels and semen analysis. J Urol 2012;188:1429-35. [PubMed]