Fertility preservation for boys and adolescents facing sterilizing medical therapy
Introduction
Advances in cancer treatment have led to increased survival of children with cancer, with 5-year survival rates of all childhood cancers improved from 58% for cases diagnosed between 1975 and 1979 to 83% for cases diagnosed during 2003 through 2009 (1). Childhood cancer survival rates differ by cancer type and patient age ranging from 67% for rhabdomyosarcoma, 71% for osteosarcoma, 72% for brain and CNS tumors, 78% for neuroblastoma, 87% for non-Hodgkin lymphoma (NHL), 89% for acute lymphocytic leukemia (ALL), 90% for Wilms tumor, 97% for Hodgkin’s disease, and 98% for retinoblastoma (2). Although cure and survival remain the top priority for the child and parents, fertility after treatment has increasingly become more important given the high survival rate. Treatments should maintain comprehensive cancer care goals and consider the long-term quality of life of these children. Minimizing the “cost of cure” remains a challenge to healthcare providers (3). The increasing numbers of childhood cancer survivors has spurred increasing interest in the early and late effects of treatment, including infertility (4).
Effect of cancer treatment on the fertility of children
Anticancer treatment, in the form of surgery, cytotoxic chemotherapy, novel targeted therapy, immunological therapy, and radiotherapy may have detrimental effects on the testis at all stages of life. They can cause persistent or long lasting damage to germ cells, somatic cells critical to germ cell survival and maturation such as Sertoli cells, and Leydig cells, critical for testosterone production. The degree of damage sustained by the testicles depends on several factors; the type of cancer, patients’ age, treatment modality, type and dosage of chemotherapy, and dose and fractionation schedule of radiotherapy (5). Animal studies suggest that cytotoxic therapy disrupts spermatogenesis by targeting rapidly dividing cells such as spermatogonial stem cells (SSC) (6).
The extent of testicular damage by radiotherapy depends on several factors, including the field of treatment, total dose, and the fractionation schedule. Direct irradiation of the testicles, as in cases of leukemic infiltration of the testes, and whole body irradiation (prior to bone marrow transplantation), directly damages the testis. Cranial irradiation of brain tumors may cause disruption of the hypothalamic-pituitary-gonadal axis and endocrine failure (7). Testicular dysfunction and impaired spermatogenesis occur with doses as low as 1.2 Gy, and irreversible damage starts with doses of 4 Gy or more. Leydig cell function is usually maintained for doses up to 20 Gy. Therefore, damage may occur to the gonads while the child grows and develops normal secondary sexual characteristics, but presents as an adult with infertility secondary to oligospermia or azoospermia (8,9). Although fractionation may be beneficial of reducing the dosage per session, it reduces the time available for tissue healing and repair (10).
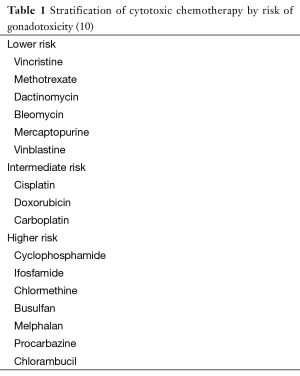
Damage by cytotoxic chemotherapy depends on total dose, type of chemotherapy and age of the patient (11,12). In addition, most chemotherapy protocols, which usually involve the use of combinations of cytotoxic chemotherapeutic agents, may have synergistic hazardous effect on fertility (13,14). Cytotoxic chemotherapy, similar to radiotherapy, affects mainly the germ cells, causing oligospermia or azoospermia. Leydig cell functions are generally not affected except with very high cumulative doses (15). The risk of infertility posed by chemotherapy varies; however, alkylating agents such as cyclophosphamide and busulfan are among the worst (Table 1) (10,16). However, it remains difficult to quantify the exact infertility risk posed by each individual drug, as most are administrated as a part of multidrug regimens (10).
In the 2013, the American Society of Clinical Oncology (ASCO) highlighted the shortage of available evidence regarding the effect of targeted therapies on male fertility (17). Tyrosine kinase inhibitors (TKIs) have revolutionized therapy for chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) turning these conditions from deadly to chronic diseases. Their role in other malignancies and non-malignant conditions is under investigation and their use has been rapidly expanding (18-20). Imatinib has recently been approved for use in children with acute lymphoblastic leukemia (ALL). TKIs may cause oligozoospermia (21,22) or affect critical functional properties of sperm such as capacitation and the acrosome reaction. Despite very limited evidence, the risk of impaired fertility should be considered and pretreatment sperm cryopreservation should be discussed. More studies are warranted to clarify the effects of targeted therapy on the male fertility (23).
Surgical treatment for childhood cancers may also affect fertility. Testicular cancer is associated with impaired semen parameters (e.g., decreased sperm concentration and sperm motility, total motile count), which may be further aggravated by orchiectomy (24). Moreover, retroperitoneal surgeries, as retroperitoneal lymph node dissection in cases of testicular cancer, or resection of retroperitoneal sarcomas can cause infertility by damaging the nerves responsible for ejaculation, causing retrograde ejaculation or anejaculation (25). However, nerve sparing surgical techniques substantially decrease the risk of ejaculatory dysfunction (26).
Effect of bone marrow and stem cell transplant
For many malignancies and some benign conditions (e.g., refractory thalassemia, sickle cell anemia, Wiskott-Aldrich syndrome), bone marrow transplantation and stem cell transplantation are necessary disease curing therapies. Worldwide, an estimated 50,000-60,000 of these transplants are performed annually with 60% occurring in patients of reproductive age (27). During these transplants, the diseased bone marrow is first destroyed with chemotherapy and/or radiation therapy and then replaced with highly specialized stem cells that develop into healthy bone marrow. Administration of cytotoxic chemotherapy and radiotherapy caused infertility in greater than two thirds of pediatric patients who had received allogeneic stem cell transplant (28).
Direct harmful fertility effect of malignancy and selected benign conditions
Cancer itself may impair fertility. More than two-thirds of patients with Hodgkin’s disease have impaired semen quality (i.e., significantly lower than normal sperm concentration or motility) prior to initiation of the treatment. Pain and constitutional manifestations (e.g., fever and anorexia) have been associated with impaired semen parameters (29). Testicular cancer and extragonadal germ cell tumors have demonstrated impaired semen parameters prior to therapy. Patients with non-malignant hematological disorders, such as thalassemia and sickle cell anemia, are often treated with repeated blood transfusions leading to iron deposition in the pituitary gland (leading to central hypogonadism) (30-32) and in the testicles (causing direct testicular toxicity and abnormal semen parameters) (33-35).
Is it possible to quantify testicular damage in childhood?
While fertility preservation is very important to survivors of childhood cancer survivors (36), these boys are much less likely than their peers to have children. While this observation is often directly related to impaired fertility, other factors, including the inability to maintain a long-term relationship, fear of cancer recurrence, and fear of death from cancer can strongly impact the decision to seek fatherhood (37,38). Assessment of fertility damage remains problematic in childhood. For post-pubertal males, semen analysis represents a good indicator of spermatogenesis and testicular function, and allows for sperm cryopreservation. This is not the case for prepubertal children or those who are unable to produce an ejaculate. Assessment primarily relies on the development of secondary sexual characteristics, including testicular and penile size, as well as the presence of pubic and axillary hair. Clinical examination may show soft testes of diminished size (39). However, many patients can have severely impaired spermatogenesis but retain normal Leydig cell function and testosterone levels. Inhibin B, secreted by Sertoli cells, may be a promising indicator of diminished sperm production as a result of cytotoxic chemotherapy (40).
Methods for fertility preservation in children and adolescents facing sterilizing therapy
Advancements in semen cryopreservation in conjunction with in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) have revolutionized the options for fertility preservation in post-pubertal male children (41) and cryopreservation of semen is a well-established method for sexually and reproductively mature boys (42,43). The quality and number of healthy sperm cryopreserved determines the reproductive options available to these boys after completing therapy. Banking as many samples as possible and referring these patients for sperm banking early in the cancer therapy process is very important.
Several options have been proposed to protect against the effects of radiotherapy. Fractionation of the radiation dose might provide benefit; however, this approach could have a detrimental effect on the testis as there will be less time available for adequate tissue repair (10). Shielding of the testicles during radiotherapy is likely the most effective approach, primarily limited by patient anatomy and the required radiation field (44). Another proposed method is surgical relocation of the gonads away from the radiated field, for instance to the thighs or outside the abdominal wall (45). However, the effectiveness of these methods remains questionable (46).
Future fertility preservation options
Spermatogenesis starts before puberty and spermarche, the beginning of mature sperm production, often precedes the ability to ejaculate. Sperm may be detected in urine samples from these boys (47,48), generally occurring around age 12 or 13 (49). Therefore, it may be possible to obtain sperm from peripubertal boys by rectal electrical stimulation under anesthesia (50) or using sperm retrieval techniques such as epididymal and testicular sperm aspiration (3). Sperm obtained and cryopreserved from these techniques can be used later with IVF and ICSI, allowing for successful fertilization even in cases of severe oligospermia.
For boys who have not yet reached spermarche, investigational options provide significant hope for the future. These techniques rely upon the isolation and cryopreservation of SSCs from the prepubertal testicle prior to chemotherapy. This technique requires a testicular biopsy and cryopreservation of either whole tissue or isolated cells. After the patient is disease free, this tissue or cells may be thawed and used for induction of in vitro spermatogenesis or autologous transplantation into the patient’s own testes.
In vitro maturation of cryopreserved testicular tissue is a promising strategy to grow mature sperm for prepubertal boys. Demonstrating the feasibility of this approach, in a neonatal mouse model, testicular tissue was harvested, cryopreserved, thawed, and cultured with resulting complete spermatogenesis IVF with ICSI was performed and two generations of healthy offspring were observed. The success of this technique in mice suggests that this organ culture method may be modified for application in other mammalian species, providing a solid technical base for use in fertility preservation for prepubertal boys facing sterilizing chemotherapy (51,52). Preliminary results using human SSC suggest that it may be possible to induce meiotic differentiation; however, arrest of maturation occurred and mature spermatozoa have not been observed (53,54).
Autologous SSC transplant is an exciting technique that has demonstrated success in restoring spermatogenesis in many non-primate models for more than 15 years (55-63) and, most recently, in primates (64). Two potential transplantation approaches are possible: either using a mixture of testicular cells containing all cell types found in the testicle, or using specific populations of isolated testicular cells. Whole cell testicular cell transplants have led to viable embryos and offspring in a number of non-primate and rhesus macaque models (55,64,65). No increased risk of developmental or epigenetic defects in these offspring has been reported (66). However, a significant potential risk of this approach is the risk of reintroduction of malignant cells back to the patient.
Purification, isolation, and in vitro growth of SSC remain a core challenge of the development of the technique to transplant specific cell populations (67). It is neither yet possible to transplant a pure population of SSC in primates nor known precisely which cells are the most important for restoring spermatogenesis. A significant advantage of transplanting isolated, purified SSC and other cells populations critical for spermatogenesis would be a much lower risk of malignant cell transfer. This risk is particularly important for boys with hematological malignancies such as leukemia and solid tumors with possible spread to the testicle, particularly given that the process of germ cell harvesting would be done prior the initiation of treatment (68,69).
Although hormonal suppression has been proposed as a fertility preservation technique in children, the approach has not demonstrated success. Hypothetically, the down-regulation of cellular division and maturation of testicular cells would render testicular cells less sensitive to the toxic effects of chemotherapy. However, hormonal suppression does not necessarily inhibit germ cell division but rather cells already undergoing meiotic divisions. Because of these findings, this approach is not recommended (70).
Potential risks to offspring from cancer treatment
As SSC are not abundant in the prepubertal testes, relatively large biopsies may be needed for future fertility preservation techniques, which could result in reduced testicular function and hypogonadism (71). Another important issue is the risk of mutations induced by the cytotoxic treatment. It is well established that some cancers have a genetic predisposition (e.g., Li-Fraumeni, retinoblastomas) (72). However, sporadic cancers, including most pediatric malignancies, are thought to arise from low penetrance gene-environment interactions (73). Although offspring of cancer survivors have not had an increased risk of developing cancer or congenital anomalies than the general population, these observations have been made in offspring of natural conception rather than men using ARTs (74,75). Selection of sperm with IVF/ICSI bypasses natural barriers to conception with an unknown risk of congenital anomalies for cancer patients.
Role of healthcare providers
The ASCO has recommended that healthcare providers discuss the risks of cancer therapy to their patients’ fertility and offer fertility preservation referral (17). Even with this recommendation, many barriers to the utilization of fertility preservation options by young patients with cancer exist including limited or nonexistent insurance coverage to address this complication of cancer therapy, inadequate access to fertility specialists, language barriers, and knowledge gaps among providers and patients. ASCO has also recommended that additional well-designed studies evaluating methods of fertility preservation and research addressing the comparative effectiveness of different methods of fertility preservation be performed.
Oncologists appropriately focus on survival when counseling their patients and their parents about the different possible treatment strategies. With the great improvements in childhood cancer treatment and survival allowing for the growing interest in quality of life after therapy, including their ability to father children, physicians should consider the risk of infertility of the proposed treatment, and possible ways to minimize this risk. Furthermore, if significant fertility risk from cancer therapy exists, patients should be informed of fertility preservation methods and be referred promptly to a fertility specialist (5). While in many cases cancer treatment must occur soon after diagnosis, fertility preservation rarely delays cancer therapy. From a fertility preservation perspective, having enough time to bank one or more semen samples and planning for possible surgical procedures is critical. Future fertility treatments like intrauterine insemination (IUI) often require at least ten million viable cryopreserved sperm to be successful. Semen samples from cancer patients will not uniformly contain this amount of sperm requiring banking of multiple samples or future use of IVF. Banking as many samples as possible keeps options open for each patient. Rapid referral to a fertility specialist allows implementation of all fertility preservation approaches.
This rapid referral also allows patients and their families enough information to understand the long-term risk to their fertility. Cancer survivors have reported that fertility was rarely discussed prior to treatment and was more often considered during the course of treatment or while learning of possible long term treatment consequences (76). These observations likely vary by medical center and reflect the availability of fertility preservation specialty services and knowledge of these services. Interestingly, many cancer survivors report they would have opted for any experimental method of fertility preservation if offered at the time of cancer diagnosis (76). Patients and parents may not be aware of the possibility of infertility or may be too overwhelmed by the cancer diagnosis and prognosis to process this side effect (77). In addition, many patients may not be aware of the possibility of fertility preservation or believe that fertility preservation may cause a delay in cancer treatment. Therefore, the medical team should acknowledge the risk of infertility at cancer diagnosis and make this risk clear to patients and parents (76). Hospitals that develop comprehensive teams of pediatric oncologists, reproductive urologists and endocrinologists, social workers, and nursing staff would likely improve fertility preservation outcomes for these vulnerable patients.
Costs: who pays?
To date, testicular tissue and semen cryopreservation are not yet standard practice in most pediatric oncology centers. For some families, failure of insurance to cover fertility preservation makes these services inaccessible. The estimated cost of preserving three semen samples, obtained by masturbation, for 3 years was $1,500 (46), costs borne in most cases by the patients or their families. Utilization of this sperm may require IVF/ICSI during adulthood, an approach that, in today’s dollars, can cost a patient over $20,000 (78). Although some hospital programs have funds to cover the costs of harvesting and storage of testicular tissue, more often these are costs paid by patients. An additional barrier is that very few cancer centers offer testicular tissue cryopreservation for young boys, primarily due to uncertainties about the potential to use this tissue in the future. Healthcare providers should encourage their patients to explore their insurance coverage and investigate advocacy programs such as Livestrong, Fertile Hope, and other charitable organizations to identify financial assistance. Many sperm banks facilitate the process of payment by offering monthly payment plans, which may make it more affordable (17).
Ethical challenges
Preservation of fertility in children and adolescents raises several important ethical questions. The challenge is in balancing the potential benefit to a prepubertal boy facing sterilization by cryopreserving testicular tissue before chemotherapy when the benefits of this approach remain theoretical. Animal studies demonstrate significant promise including studies in primates; however, no human studies have yet demonstrated success with in vitro maturation of sperm, a critical step toward using testicular tissue for IVF, nor demonstrated success with testicular cell transplantation and resumption of normal spermatogenesis. Should boys be subjected to a testicular biopsy with its potential for surgical risk when the risk of sterilization is very high or should families skip this experimental approach and hope for the best? For post-pubertal or prepubertal boys, generally older than 12 years old, semen cryopreservation or surgical sperm retrieval are established, standard clinical therapies utilized regularly by reproductive urologists. For all of these children, while survival probability has improved significantly over the past several decades, the risk of death remains. Disposition of cryopreserved tissue is an important consideration. For boys under 18, only two options should be considered: discarded upon death of the patient or designated for research use. Posthumous reproduction is not an ethical option for children (5). Obtaining informed consent from parents and assent from younger boys is critically important (79). Interventions to preserve fertility should not raise unrealistic expectations or have long-term adverse effects on the patients or their offspring (80). In general, ethical fertility preservation research involving children should be (81):
- Scientifically valid;
- In the patient’s best interest;
- Subject to individual ethical review;
- Subject to informed consent;
- Supported by animal model alternatives before involving children;
- Led by physicians with the necessary expertise and facilities to carry out the research.
Ethical challenges for prepubertal boys
Methods of fertility preservation involving testicular biopsies and cryopreservation for prepubertal children are still experimental and should be conducted under Institutional Review Board (IRB) guidance. Since these biopsies may ultimately prove beneficial for these children, they may be done with the boy’s formal assent (if under 18) in addition to the parental consent. While the potential enormous benefit of future fertility outweighs the small risks of testicular biopsy prior to cancer treatment (5), successful development of fertility restoration in humans may take decades or never be achieved (80). However, if the testicular biopsy does not occur prior to cancer treatment, the child may lose his best opportunity to protect his fertility.
Ethical challenges for post-pubertal boys
Post-pubertal boys will often be able to ejaculate and provide sperm for cryopreservation. Nevertheless, it is important to discuss this option with them in a comfortable setting, including discussions outside of the presence of their parents. For most families, semen obtained by ejaculation usually poses few ethical problems though religious and moral objections sometimes limit this approach (82). For sperm cryopreservation, parental consent is required (83).
Conclusions
Improvements in childhood cancer survival have allowed boys and their families to increasingly focus on quality of life after therapy, particularly their future ability to father children. Semen cryopreservation is a well-established method of fertility preservation for post-pubertal children. On the other hand, the use of cryopreserved testicular tissue represents a promising, yet experimental method of fertility preservation for prepubertal males facing sterilizing therapy that should be offered only in IRB approved settings. Healthcare providers should counsel families about the fertility risks of therapy, discuss or refer patients for standard fertility preservation options, and consider experimental approaches to fertility preservation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute, 2013.
- Grundy R, Gosden RG, Hewitt M, et al. Fertility preservation for children treated for cancer (1): scientific advances and research dilemmas. Arch Dis Child 2001;84:355-9. [PubMed]
- Stevens MC, Mahler H, Parkes S. The health status of adult survivors of cancer in childhood. Eur J Cancer 1998;34:694-8. [PubMed]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril 2005;83:1622-8. [PubMed]
- Meistrich ML, Finch M, da Cunha MF, et al. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res 1982;42:122-31. [PubMed]
- Littley MD, Shalet SM, Beardwell CG, et al. Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 1989;31:363-73. [PubMed]
- Shalet SM, Tsatsoulis A, Whitehead E, et al. Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol 1989;120:161-5. [PubMed]
- Castillo LA, Craft AW, Kernahan J, et al. Gonadal function after 12-Gy testicular irradiation in childhood acute lymphoblastic leukaemia. Med Pediatr Oncol 1990;18:185-9. [PubMed]
- Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol 2005;6:209-18. [PubMed]
- Waring AB, Wallace WH. Subfertility following treatment for childhood cancer. Hosp Med 2000;61:550-7. [PubMed]
- Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol 1999;33:2-8. [PubMed]
- Clayton PE, Shalet SM, Price DA, et al. Testicular damage after chemotherapy for childhood brain tumors. J Pediatr 1988;112:922-6. [PubMed]
- Meistrich ML, Wilson G, Brown BW, et al. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer 1992;70:2703-12. [PubMed]
- Gerl A, Mühlbayer D, Hansmann G, et al. The impact of chemotherapy on Leydig cell function in long term survivors of germ cell tumors. Cancer 2001;91:1297-303. [PubMed]
- Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update 2004;10:251-66. [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500-10. [PubMed]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996;2:561-6. [PubMed]
- Blay JY. A decade of tyrosine kinase inhibitor therapy: Historical and current perspectives on targeted therapy for GIST. Cancer Treat Rev 2011;37:373-84. [PubMed]
- Kontzias A, Laurence A, Gadina M, et al. Kinase inhibitors in the treatment of immune-mediated disease. F1000 Med Rep 2012;4:5. [PubMed]
- Mariani S, Basciani S, Fabbri A, et al. Severe oligozoospermia in a young man with chronic myeloid leukemia on long-term treatment with imatinib started before puberty. Fertil Steril 2011;95:1120.e15-7.
- Seshadri T, Seymour JF, McArthur GA. Oligospermia in a patient receiving imatinib therapy for the hypereosinophilic syndrome. N Engl J Med 2004;351:2134-5. [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility Preservation for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2013;31:2500-10. [PubMed]
- Williams DH 4th, Karpman E, Sander JC, et al. Pretreatment semen parameters in men with cancer. J Urol 2009;181:736-40. [PubMed]
- Kort JD, Eisenberg ML, Millheiser LS, et al. Fertility issues in cancer survivorship. CA Cancer J Clin 2014;64:118-34. [PubMed]
- Pearce S, Steinberg Z, Eggener S. Critical evaluation of modified templates and current trends in retroperitoneal lymph node dissection. Curr Urol Rep 2013;14:511-7. [PubMed]
- Pasquini M, Wang Z. Current use and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides 2009. Available online: http://www.cibmtr.org/PUBLICATIONS/Newsletter/index.html
- Borgmann-Staudt A, Rendtorff R, Reinmuth S, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant 2012;47:271-6. [PubMed]
- Rueffer U, Breuer K, Josting A, et al. Male gonadal dysfunction in patients with Hodgkin’s disease prior to treatment. Ann Oncol 2001;12:1307-11. [PubMed]
- Bajoria R, Chatterjee R. Hypogonadotrophic hypogonadism and diminished gonadal reserve accounts for dysfunctional gametogenesis in thalassaemia patients with iron overload presenting with infertility. Hemoglobin 2011;35:636-42. [PubMed]
- De Sanctis V, Candini G, Giovannini M, et al. Abnormal seminal parameters in patients with thalassemia intermedia and low serum folate levels. Pediatr Endocrinol Rev 2011;8 Suppl 2:310-3. [PubMed]
- Agbaraji VO, Scott RB, Leto S, et al. Fertility studies in sickle cell disease: semen analysis in adult male patients. Int J Fertil 1988;33:347-52. [PubMed]
- Soliman AT, elZalabany MM, Ragab M, et al. Spontaneous and GnRH-provoked gonadotropin secretion and testosterone response to human chorionic gonadotropin in adolescent boys with thalassaemia major and delayed puberty. J Trop Pediatr 2000;46:79-85. [PubMed]
- De Sanctis V, Atti G, Lucci M, et al. Endocrine assessment of hypogonadism in patients affected by thalassaemia major. Ric Clin Lab 1980;10:663-71. [PubMed]
- Al-Rimawi HS, Jallad MF, Amarin ZO, et al. Pubertal evaluation of adolescent boys with beta-thalassemia major and delayed puberty. Fertil Steril 2006;86:886-90. [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 2002;20:1880-9. [PubMed]
- Andersen BL. Surviving cancer: the importance of sexual self-concept. Med Pediatr Oncol 1999;33:15-23. [PubMed]
- Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: a review. Med Pediatr Oncol 1999;33:53-9. [PubMed]
- Siimes MA, Rautonen J. Small testicles with impaired production of sperm in adult male survivors of childhood malignancies. Cancer 1990;65:1303-6. [PubMed]
- Crofton PM, Thomson AB, Evans AE, et al. Is inhibin B a potential marker of gonadotoxicity in prepubertal children treated for cancer? Clin Endocrinol (Oxf) 2003;58:296-301. [PubMed]
- Tournaye H, Goossens E, Verheyen G, et al. Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update 2004;10:525-32. [PubMed]
- Müller J, Sonksen J, Sommer P, et al. Cryopreservation of semen from pubertal boys with cancer. Med Pediatr Oncol 2000;34:191-4. [PubMed]
- Yee S, Fuller-Thomson E, Dwyer C, et al. “Just what the doctor ordered”: Factors associated with oncology patients’ decision to bank sperm. Can Urol Assoc J 2012;6:E174-8. [PubMed]
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917-31. [PubMed]
- Acosta JM, Tiao G, Stein JE, et al. Temporary relocation of testes to the anterior abdominal wall before radiation therapy of the pelvis or perineum. J Pediatr Surg 2002;37:1232-3. [PubMed]
- Fallat ME, Hutter J. American Academy of Pediatrics Committee on Bioethics, et al. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics 2008;121:e1461-9. [PubMed]
- Nielsen CT, Skakkebaek NE, Richardson DW, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab 1986;62:532-5. [PubMed]
- Kulin HE, Frontera MA, Demers LM, et al. The onset of sperm production in pubertal boys. Relationship to gonadotropin excretion. Am J Dis Child 1989;143:190-3. [PubMed]
- Hirsch M, Lunenfeld B, Modan M, et al. Spermarche--the age of onset of sperm emission. J Adolesc Health Care 1985;6:35-9. [PubMed]
- Schmiegelow ML, Sommer P, Carlsen E, et al. Penile vibratory stimulation and electroejaculation before anticancer therapy in two pubertal boys. J Pediatr Hematol Oncol 1998;20:429-30. [PubMed]
- Sato T, Katagiri K, Kubota Y, et al. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc 2013;8:2098-104. [PubMed]
- Yokonishi T, Sato T, Komeya M, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 2014;5:4320. [PubMed]
- Nowroozi MR, Ahmadi H, Rafiian S, et al. In vitro colonization of human spermatogonia stem cells: effect of patient’s clinical characteristics and testicular histologic findings. Urology 2011;78:1075-81. [PubMed]
- Huleihel M, Abuelhija M, Lunenfeld E. In vitro culture of testicular germ cells: regulatory factors and limitations. Growth Factors 2007;25:236-52. [PubMed]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994;91:11303-7. [PubMed]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994;91:11298-302. [PubMed]
- Schlatt S, Rosiepen G, Weinbauer GF, et al. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod 1999;14:144-50. [PubMed]
- Honaramooz A, Behboodi E, Blash S, et al. Germ cell transplantation in goats. Mol Reprod Dev 2003;64:422-8. [PubMed]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod 2002;66:21-8. [PubMed]
- Izadyar F, Den Ouden K, Stout TA, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction 2003;126:765-74. [PubMed]
- Mikkola M, Sironen A, Kopp C, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim 2006;41:124-8. [PubMed]
- Herrid M, Olejnik J, Jackson M, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod 2009;81:898-905. [PubMed]
- Kim Y, Turner D, Nelson J, et al. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction 2008;136:823-31. [PubMed]
- Hermann BP, Sukhwani M, Winkler F, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012;11:715-26. [PubMed]
- Shinohara T, Inoue K, Ogonuki N, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod 2002;17:3039-45. [PubMed]
- Goossens E, De Rycke M, Haentjens P, et al. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod 2009;24:2255-63. [PubMed]
- Smith JF, Yango P, Altman E, et al. Testicular Niche Required for Human Spermatogonial Stem Cell Expansion. Stem Cells Transl Med 2014;3:1043-54. [PubMed]
- Shaw JM, Bowles J, Koopman P, et al. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod 1996;11:1668-73. [PubMed]
- Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod 1997;12:403-5. [PubMed]
- Thomson AB, Anderson RA, Irvine DS, et al. Investigation of suppression of the hypothalamic-pituitary-gonadal axis to restore spermatogenesis in azoospermic men treated for childhood cancer. Hum Reprod 2002;17:1715-23. [PubMed]
- Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997;12:1688-92. [PubMed]
- Malkin D, Portwine C. The genetics of childhood cancer. Eur J Cancer 1994;30A:1942-6. [PubMed]
- Shields PG, Harris CC. Cancer risk and low-penetrance susceptibility genes in gene-environment interactions. J Clin Oncol 2000;18:2309-15. [PubMed]
- Hawkins MM, Draper GJ, Smith RA. Cancer among 1,348 offspring of survivors of childhood cancer. Int J Cancer 1989;43:975-8. [PubMed]
- Li FP, Fine W, Jaffe N, et al. Offspring of patients treated for cancer in childhood. J Natl Cancer Inst 1979;62:1193-7. [PubMed]
- Nieman CL, Kinahan KE, Yount SE, et al. Fertility preservation and adolescent cancer patients: lessons from adult survivors of childhood cancer and their parents. Cancer Treat Res 2007;138:201-17. [PubMed]
- Kazak AE, Boeving CA, Alderfer MA, et al. Posttraumatic stress symptoms during treatment in parents of children with cancer. J Clin Oncol 2005;23:7405-10. [PubMed]
- Wu AK, Odisho AY, Washington SL 3rd, et al. Out-of-pocket fertility patient expense: data from a multicenter prospective infertility cohort. J Urol 2014;191:427-32. [PubMed]
- Ondrusek N, Abramovitch R, Pencharz P, et al. Empirical examination of the ability of children to consent to clinical research. J Med Ethics 1998;24:158-65. [PubMed]
- Grundy R, Larcher V, Gosden RG, et al. Fertility preservation for children treated for cancer (2): ethics of consent for gamete storage and experimentation. Arch Dis Child 2001;84:360-2. [PubMed]
- McIntosh N, Bates P, Brykczynska G, et al. Guidelines for the ethical conduct of medical research involving children. Royal College of Paediatrics, Child Health: Ethics Advisory Committee. Arch Dis Child 2000;82:177-82. [PubMed]
- Babayev SN, Arslan E, Kogan S, et al. Evaluation of ovarian and testicular tissue cryopreservation in children undergoing gonadotoxic therapies. J Assist Reprod Genet 2013;30:3-9. [PubMed]
- English V, Mussell R, Sheather J, et al. Review of the Human Fertilisation and Embryology Act 1990. J Med Ethics 2005;31:743-4.