Do endocrine disruptors cause hypospadias?
Background
Endocrine disruptors are defined as “chemicals that may interfere with the body’s endocrine system and produce adverse developmental, reproductive, neurological, and immune effects in both humans and wildlife” by the National Institute of Environmental Health Sciences (NIEHS), a division of the National Institute of Health (NIH) (1). There has been a progressively increasing concern over such agents in the environment and the effects they may have. Diethylstilbestrol (DES) represents an example with adverse long-term outcomes, namely reproductive tract carcinogenesis and teratogenesis (2).
Although there have been such historic precedents, this phenomenon did not become labelled until quite recently. A PubMed literature search for “endocrine disruptor” yields the earliest result was in 1996. A few years prior, Theo Colborn, an environmental biologist studying the risks of synthetic chemicals in the Great Lakes area, organized the Wingspread Conference in 1991. These discussions amongst experts from disparate fields culminated in a consensus statement. It was here that the term, “endocrine disruptor”, was coined (3). In 1996, Colborn published Our Stolen Future, illustrating her concerns regarding endocrine disruption due to environmental agents.
Correspondingly, in 1996, the United States Congress passed the Food Quality Protection Act (FQPA) and amended the Safe Drinking Water Act (SDWA) to address endocrine disruption. These legislations are provisions which charged the Environmental Protection Agency (EPA) to call for the screening and testing of chemicals and pesticides for possible endocrine disrupting effects (4). Under this mandate, the EPA established the Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) to advise on execution of these mandates. On recommendation from the EDSTAC, the EPA has established a two-tiered screening and testing process: Tier 1—“..to identify chemicals that have the potential to interact with the endocrine system”; Tier 2—“..determine the endocrine-related effects caused by each chemical and obtain information about effects at various doses” (5).
Since these policy decisions, there has been a progressive increase in studies investigating endocrine disruptors. These studies have focused on various organ systems, including the genitourinary tract, whose development is particularly influenced by a delicate local and systemic hormonal balance.
Hypospadias is a common genitourinary anomaly that has been extensively studied, yet the exact etiology remains uncertain. Concurrent with the increase in industrial chemical agents, there have been numerous studies reporting an increasing prevalence of hypospadias in humans (6-8). Thus, there has been concern that agents in the environment acting as endocrine disruptors are causing this rise in hypospadias.
Herein, we review the current literature in hopes to answer the question of whether endocrine disruptors cause hypospadias.
Methods
A literature search was performed using the PubMed database for the terms “Endocrine disruptor” or “Endocrine disruptors” and “Hypospadias”. Search results included recent articles within the past 5 years as well as additional relevant and seminal articles on the topic. Studies included both animal and human models. Studies were individually reviewed and presented. The results of the current literature were classified as the following different types of studies:
- Basic Science studies;
- Clinical Science studies;
- Epidemiologic studies;
- Review articles.
Relevant sentinel articles were also reviewed. Additionally, a few pertinent studies were extracted from the reference of the articles obtained from initial search results.
Results
Endocrine disruptors and their relationship to hypospadias have been extensively investigated in recent years. Initial concern was sparked with the reported rise in the prevalence of hypospadias in humans. Multiple approaches have been used since then. The initial wave of studies utilized an epidemiologic approach via cohort and case-control studies to establish an association between environmental exposure and human pathology. Basic science studies have further been performed by studying animals treated perinatally with known endocrine-disrupting agents. Various animal models have provided a means to explore molecular mechanisms. Human studies consist of correlative epidemiologic studies.
Hypospadias definition
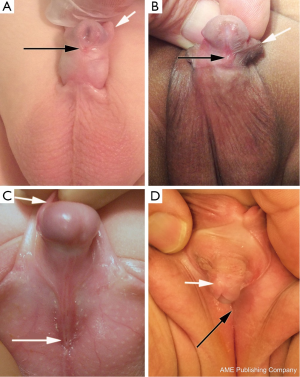
Hypospadias is a common genitourinary malformation that is diagnosed on physical examination in humans. The abnormality presents as a wide spectrum of findings (Figure 1); however, key characteristics include a ventrally deficient prepuce and a proximal urethral meatus (9). Additional abnormal findings may include downward glans tilt, deviation of the median penile raphe, penile curvature, and scrotal encroachment onto the penile shaft, midline scrotal cleft, and penoscrotal transposition (9).
Epidemiology
The prevalence of hypospadias has generally been reported as occurring approximately 1 in 300 male children (9). However, in recent years, there have been reports that the prevalence of hypospadias has been increasing. In the 1980s, hypospadias was reported to have a statistically significant increase in the Hungarian congenital malformation registry (7). Another study was performed in the United States in 1997 comparing the Metropolitan Atlanta Congenital Defects Program (MACDP) and the Birth Defects Monitoring Program (BDMP), a registry of newborn discharge diagnoses from a nationwide sample of hospitals. Hypospadias prevalence from 1970 to 1993 had nearly doubled from 20.2 to 39.7 per 10,000 (8). However, in regard to this apparent increase in the incidence of human hypospadias concern has been voiced on whether the “increase in hypospadias is confounded by diagnostic criteria” (10).
Accordingly, recent studies contest the idea of an increase in the incidence of human hypospadias. A retrospective review of the New York State Congenital Malformations Registry from 1983 to 2005 demonstrated a decrease in the rate of hypospadias from 36.34 per 10,000 live births in 1983 to a rate of 34.9±0.36 per 10,000 live births in the period 1992-2005 (10). Data from 1984 to 1997 within the California March of Dimes Birth Defects Foundation/California Birth Defects Monitoring Program demonstrated that hypospadias prevalence had not increased during the 13-year study period (11). Data from Washington State do not demonstrate a significant change in the prevalence of hypospadias from 1987 to 2002 (12).
Studies of hypospadias
Hypospadias is a well-described congenital birth defect historically dating to Greek and Roman cultures. Studies on humans by nature are limited to observational, surgical and genetic studies with obvious experimental limitations. This has made the development of a relevant animal model all the more critical. Based on the ability to manipulate the genetic make-up, the short gestation period (~20 days) and length of penile maturation (~20 days post natal), the murine model has been a natural choice. Despite the common use of the murine model, there are obvious differences in anatomy of mouse and human penis that need to be taken into consideration.
Comparison of penile anatomy in mouse and human
Penile nomenclature between mice and humans is quite different and must be thoroughly understood to avoid confusion. In both mouse and human, a portion of the penis lies below the body surface (internal) and a portion projects from the body wall (external). In humans the internal portion of the penis is comprised of the proximal attachments of the corpora cavernosa and corpus spongiosum. The external portion termed the shaft or body of the penis includes the corporal body and corpus spongiosum. The distal corpus spongiosum expands to form the glans, which is small relative to size of the shaft.
In the mouse, the internal portion of the penis is called the body, containing the corporal body and its attachments to the pubic bones. Unlike the human penis, the external portion of the mouse penis lies entirely within the preputial space, is called the glans, and contains multiple erectile bodies as well as the os penis. The mouse glans penis is relatively long with a shaft proximally and a specialized region distally comparable to the human glans. Thus, the mouse glans as well as the pendulous human penis is both external projections of the body wall and the predominant site of hypospadias in both species.
Basic science studies
Recognizing homologous anatomy, the murine model has become the common choice for investigation of hypospadias. Animal models have been used to explore the effects of endocrine disruptors on external genitalia. Yucel et al. demonstrated that estrogen arrests murine urethral seam formation, which occurs by fusion of the epithelial edges of the urethral folds. Also, androgens were shown to accelerate murine urethral seam formation (13). Kim et al. showed how maternal estrogen exposure disrupted the urethral seam in male offspring causing hypospadias (14). Vinclozolin exposure to pregnant dams resulted in males with hypospadias, while females developed longer urethras. Females also exhibited up-regulation of progesterone receptor (PR) mRNA with vinclozolin exposure (15). Willingham indicated that Loratadine, a common medication for allergies, disrupted urethral development and steroid receptor expression (16). Agras demonstrated that estrogen upregulates expression of androgen receptor, progesterone receptor, and ERα expression in the murine genital tubercle of males and females; in contrast, estrogen receptor-beta (ERβ) did not demonstrate any differences between genders during fetal life (17-19). Benzophenone-2, an additive in cosmetic products and food container plastics, was suggested as a possible cause for hypospadias as embryonic genital tubercles of treated mice exhibited an open urethral groove, indicative of failure of fusion of the preputial/urethral folds (20). Similarly, maternal oral exposure to genistein and vinclozolin elicited a 25% and 42% rate of hypospadias both macroscopically and histologically (21). Finally, Yong showed the role for chaperone molecules in androgen-receptor mediated signaling with respect to penile hypospadias and prostate dysgenesis (22).
The environment
Zhang et al. investigated rats treated in utero via maternal oral exposure to Di-n-butyl phthalate (DBP), an agent within polyvinyl chloride plastics (23). The offspring of timed-pregnant rats exposed to DBP exhibited decreased B-catenin expression in the genital tubercle as judged by immunohistochemistry, implicating the Wnt/B-catenin pathway in genital tubercle development. Rat models have also been employed as part of a broader methodology to assess for endocrine disruption. For instance, the International Life Sciences Institute’s (ILSI) Agricultural Chemical Safety Assessment (ACSA) project has developed a testing scheme to develop a consensus approach for assessing safety of crop protection products. Schneider et al. utilized a rat model following the ILSI-ACSA methodology to study the effects of Vinclozolin in developing rats (24). Dosing began prior to mating and continued to F1 generation weaning. The low-dose and high-dose treatment groups exhibited reduced anogenital distance. At the high-dose, treatment groups exhibited penile hypospadias, purulent prostatitis, seminal vesicle inflammation, and F1 females exhibited accelerated vaginal opening.
Similarly, murine models have also been utilized. Ross et al. demonstrated the effects of genistein, a phytoestrogen in soy, on the development of the male mouse urethra (25). Microarray analysis of urethral tissue demonstrated altered gene expression in 277 genes. Spanning across multiple genetic signaling cascades, these alterations affected pathways of cell proliferation, adhesion, apoptosis and tubular morphogenesis (P<0.0001). Genes in the MAPK and TGF-β signaling pathways and those controlled by FOXO, HOX and ER transcription factors were particularly disrupted.
Organ culture systems have also been utilized. Ma et al. developed a genital tubercle culture system in vitro to investigate the effects of estrogenic and androgenic states on mouse penile and urethral development (26). Treatment with DHT plus high-dose estrogen elicited decreased proliferation and increased apoptosis in the mesenchyme flanking the urethral plate epithelium.
Genetics
Although there have been numerous studies, many questions remain about the genetic basis for human hypospadias. Family-based studies have demonstrated a high degree of concordance. However, these results are not uniform; rather, there appears to be variations in the penetrance of human hypospadias. Thus, other factors beyond solely genetics may be at play. It may very well be that human hypospadias has a multifactorial etiology of polygenic inheritance in combination with simultaneous response to environmental agents. Nonetheless, genetic studies have largely focused on identification of candidate genes involved in genital tubercle development and/or steroid metabolism.
The interaction between environmental factors and genetic factors was investigated by Qin et al. by using human foreskin fibroblasts (27). On exposure to Bisphenol A (BPA), genital fibroblasts from hypospadias patients exhibited reduced expression of matrix metalloproteinase-11. The effects of single-nucleotide polymorphisms (SNPs) were also investigated amongst the specimens, with BPA-treatment groups demonstrating decreased NTSR1 expression.
The role of these SNPs in metabolism of endocrine disruptors was further studied in two populations, patients from Japan and Italy. Qin et al. showed that polymorphisms were found to be significantly associated with congenital malformations, including hypospadias. Two specific polymorphisms demonstrated an approximately 70% prediction accuracy for hypospadias in the Japanese population (P=0.001) (28). These SNPs may perhaps reflect the role for genes involved in metabolism of endocrine disruptors as an etiology for hypospadias.
Others have investigated hypospadias as a component of a larger disorder, testicular dysgenesis syndrome (TDS). Comprising a set of genitourinary malformations (including hypospadias, cryptorchidism, hypospermatogenesis, and testicular cancer), this syndrome has been frequently described across the literature and suggested to be associated with genetic as well as environmental factors (29-31).
Qiao et al. examined the role of ERα and ERβ in human hypospadias tissue (32). Foreskin tissue from normal control and hypospadiac patients were analyzed to quantify ER (α and β) by mRNA expression. ER mRNA (α and β) was significantly decreased in hypospadiac patients (P<0.001). Moreover, while normal controls exhibited strong ERβ staining, hypospadiac patients exhibited weak or undetectable ERβ and weak ERα. Human fetal penis tissue samples were also analyzed via IHC. These showed strong ER-β staining with no detection of ERα. These differences in expression of ERs suggest an alteration in estrogenic effects, leading to hypospadias in humans. In the murine model, ER-α expression was shown to increase until parturition while ER-β expression did not increase. This was felt to imply genital tubercle sensitivity to estrogen increases during fetal life (19).
Interestingly, Padilla-Banks reported effects of endocrine disruptors on developing female mouse external genitalia, introducing the idea of female hypospadias (namely urethral-vaginal fistula) (33). Although the majority of hypospadias research has focused on the male external genitalia, there have also been studies reporting female hypospadias. Neonatal mice were exposed to Genistein, a phytoestrogen in soy. At 3 and 6 weeks age, examination demonstrated an abnormally positioned urethral opening. This effect was noted with complete penetrance as 10/10 animals exhibited this phenotype.
Clinical science studies
The literature search retrieved few clinical studies dealing with endocrine disruptors as a cause hypospadias perhaps because of numerous difficulties, such as scientific, financial, administrative, and ethical constraints.
However, two studies presented a novel technique in evaluation of endocrine disruption. Both studies utilized anogenital distance as an outcome of abnormal external genitalia, even though anogenital distance is not strictly a component of external genitalia. However, various animal models have historically associated reduced anogenital distance with male genital defects (34-38). Likewise, reduced anogenital distance in humans has been attributed to exposure to putative endocrine disruptors (39,40).
Hsieh et al. prospectively evaluated 119 Caucasian males, comparing anogenital distance between those with normal external genitalia or hypospadias. On age-matching analysis, hypospadiac boys exhibited a significantly shorter anogenital distance (n=26; 62±2 vs. 68±2 mm respectively, P=0.033) (41). More recently, Thankamony et al. compared anogenital distance and penile length amongst boys with cryptorchidism and hypospadias relative to normative data (42). Hypospadiac boys exhibited a shorter anogenital distance and penile length compared to unaffected controls (P<0.0001). These findings hold promise for future studies employing shortened anogenital distance and/or penile length as signals of potential endocrine disruption.
Additional studies have examined potential endocrine disruptors in serum/urine in patients with hypospadias. Choi et al. obtained urine and plasma samples from 80 controls, 80 hypospadias patients, and the mothers of 40 patients (43). The samples were analyzed for various endocrine disruptor compounds, including 5 phthalates [di-(2-ethylhexyl)phthalate (DEHP)], [di-n-butyl-phthalate (DBP), mono-(2- ethylhexyl) phthalate (MEHP), mono-n-butyl-phthalate (MBP) and phthalic acid (PA)], 2 alkylphenols [n-nonylphenol (n-NP) and t-octylphenol (t-OP)] and BPA. Urine DEHP and n-NP as well as plasma PA and BPA levels were significantly associated with hypospadias. However, maternal levels of these endocrine disruptors demonstrated no relationship to hypospadias of their offspring. Similarly, Meijer et al. examined the relationship between prenatal organo-halogen compounds (OHC) levels and male sexual development (44). Measurements included maternal OHC levels at 35 weeks gestation, serum levels of sex development-related hormones {testosterone, free testosterone, sex hormone-binding globulin (SHBG); LH, FSH, estradiol (E[2]), free E[2] (FE[2]) and inhibin B (InhB)} in their sons at 3 months of age, and neonatal testicular volume and penile length at 3 and 18 months of age. Significant associations were observed between certain OHCs and sex-hormone levels as well as testicular volume at 18 months, suggesting that these compounds may be affect developmental outcomes.
Epidemiologic studies
The majority of studies investigating endocrine disruptors in humans have utilized an epidemiologic approach. Often, cohorts are identified based on physical examination, and then retrospective analysis of exposures is performed to identify any potential association with known endocrine disruptors. While there are drawbacks to this approach, the merits remain worthwhile as an initial approach to tackling the much larger question of whether endocrine disruptors cause hypospadias.
The environment
An intriguing study by Carmichael et al. examined the associations between residential proximity to pesticide applications and hypospadias (45). Data on land use and pesticide applications were obtained, and early pregnancy exposure was determined within a 500 m radius from residence. Male infants born from 1990 to 2004 in 8 counties were included with 690 cases and 2,195 controls. Of all subjects, 41% were determined to have been exposed to 57 chemical groups and 292 agents. There were few significant associations between exposure and hypospadias, and these results were advised to be regarded with caution until replicated in other studies.
This approach of comparing primary data regarding exposures with detected hypospadias rates was utilized by Agopian et al. (46). The United States Geological Survey was used to obtain county-level exposure estimates of Atrazine, the most widely used herbicide in the United States and thought to be potential anti-androgenic endocrine disruptor. The Texas Birth Defects Registry was queried to obtain 16,433 cases with isolated male genital malformations. Population-based controls were obtained randomly from males born from 1999-2008. Logistic regressions for hypospadias demonstrated an association between medium-low and/or medium levels of estimated peri-conceptional maternal residential atrazine exposure and every male genital malformation category evaluated, corroborating prior literature of a suspected teratogenic role of atrazine, previously considered anti-androgenic.
Epidemiologic studies have also assessed maternal/fetal exposure inferred from measuring serum levels of potential endocrine disruptors in mothers of children with hypospadias. Rignell-Hydbom et al. conducted a case-control study of fetal organochloride exposure (47). The Southern Sweden Maternity Cohort (SSMC), Medical Birth Register, the Malformation Register and the In-patient Register yielded 390 SSMC mothers who had given birth to children with hypospadias from 1986-2002. Population-based controls were obtained randomly from the SSMC. Serum samples from early gestation of 270/390 mothers were measured for PCB-153, P,P’-DDE and hexachlorbenzene (HCB). Beyond certain threshold doses, P,P’-DDE and HCB yielded an elevated odds ratios (OR) [1.69, 95% confidence interval (CI), 0.97 to 2.93; 1.69, 95% CI, 0.97 to 2.93], suggesting that these compounds may be risk factors for hypospadias.
In contrast, other epidemiologic studies have established exposures based on questionnaires administered to parents. Although there remains some level of recall and selection bias, there have been many such studies. El Kholy et al. assessed stretched penile length in 1,000 prospectively enrolled Egyptian newborns, conducted interviews to assess for exposure to endocrine disruptors during pregnancy, and measured serum free testosterone levels (48). The prevalence of genital anomalies was 1.8%, with hypospadias comprising 83% of these anomalies. Endocrine disruptor exposure was noted in 81 neonates. Compared to the non-exposed group, this subset exhibited statistically significant differences with lower mean penile length (P=0.041) and serum free testosterone level (P<0.01). Moreover, the exposed-group demonstrated a significantly higher rate of anomalies P<0.0001).
Similarly, Gaspari et al. studied a unique population in northeastern Brazil where poor sanitation has encouraged widespread pesticide use (49). Over 2 years, the total birth cohort of full-term newborns from a regional hospital was analyzed. A total of 2,710 male newborns were enrolled, examined for hypospadias, cryptorchidism, and micropenis with referral for Endocrinology and Genetics workup. Utilizing an endocrinology workup, the parents of all children were interviewed for exposure to endocrine disruptors. There were 56 genital malformations detected, of which 15 were hypospadias. Endocrine disruptor exposures were noted in at least 92% of newborns with malformations.
Gaspari et al. performed a case-control study to identify risk factors for male genitalia malformations, focusing on parental prenatal exposures (50). Enrollment included 1,615 full-term newborn males, and 1,442 were examined for hypospadias, cryptorchidism, and micropenis by a single pediatrician. Nearly two controls were enrolled for each case. Parents of all newborns were interviewed for exposure to endocrine disruptors. Hypospadias comprised 14 of the 39 genital malformations. Genital malformations were significantly associated with exposure to endocrine disruptors (OR =4.41; 95% CI, 1.21-16.00). Intriguingly, familial clustering demonstrated an increased OR association with male external genital malformations (OR =7.25; 95% CI, 0.70-74.30). Thus, the data suggest that exposure to endocrine disruptors may be a potential risk factor for hypospadias.
Other investigators measured exposures to endocrine disruptors through both interviews and serum samples. In a case-control study, Giordano et al. questioned mothers of 80 hypospadiac infants and 80 healthy controls for exposures to potential endocrine disruptors (51). Serum from primiparous mothers of 37 cases and 21 controls was measured for dichlorodiphenyldichloroethylene, hexachlorobenzene, and several polychlorinated biphenyl congeners. Risk of hypospadias was associated with perinatal maternal occupational exposures to EDCs, mothers consuming a diet rich in fish or shellfish, and serum hexachlorobenzene concentration above the median of all subjects. Although the number of cases is limited, the authors conclude evidence for an association between maternal exposure to EDCs and hypospadias.
Occupational exposures to potential endocrine disruptors raise additional legal and ethical issues. Morales-Suárez-Varela et al. examined the Danish National Birth Cohort from 1997-2009 of 45,341 singleton deliveries (52). Telephone interview of parents was used to obtain information about work and job titles; a job exposure matrix for endocrine disruptors was generated. The Medical Birth and National Hospital Register provided data on congenital anomalies diagnosed at birth or during follow-up. Hypospadias incidence was reported to be 292 cases (0.6%) with a significant association to probable or possible maternal exposure to one or more endocrine disruptor [HRa =1.8 (95% CI, 1.0-2.6), HRa =2.6 (95% CI, 1.8-3.4)]. There was also a significant association to paternal exposure to heavy metals [HRa =2.2 (95% CI, 1.0-3.4)]. Thus, this study provided some evidence that occupational exposures may increase risk of hypospadias.
Similarly, Nassar et al. investigated the association between both maternal and paternal occupational exposures to EDCs and hypospadias (53). Enrollment included 1,202 cases of hypospadias and 2,583 male controls. Occupational exposures to endocrine disruptors were assessed in both parents according to a validated job-exposure matrix. Multivariable statistics demonstrated an association between maternal occupation exposure to heavy metals and phthalates and hypospadias. Paternal occupational exposure to polychlorinated organic and bi-phenolic compounds was also significantly associated.
Other epidemiologic approaches have followed patients who have been historically exposed to potential endocrine disruptors. Kalfa et al. demonstrated intriguing multi-generational effects of endocrine disruptors. Based on mouse data, exposure to DES raises the risk of genital malformation in subsequent generations (54). With a French database of 529 families with DES-exposed mothers, there was significant number of sons born to “DES-daughters” (8/97, P=0.02). Small et al. described a cohort of sons born to mothers exposed to polybrominated biphenyl (PBB), a brominated flame retardant (55). Although the 464 sons exhibited 5 cases of hypospadias, this finding was not associated with exposure to PBB. However, exposure to PBB was associated with other genitourinary pathology.
In summary, there have been a host of epidemiologic studies investigating the potential association between various exposures to endocrine disruptors and the presence of hypospadias in male offspring. The majority of these studies demonstrate significant associations, suggesting a potential environmental etiology of hypospadias.
Genetics
Although there have been numerous epidemiologic studies investigating the environmental aspects of endocrine disruption as a cause for human hypospadias, there remain signs of multifactorial etiologies through findings of familial clustering. The literature remains sparse for epidemiologic evaluation of genetic aspects of endocrine disruption.
Shekharyadav et al. investigated the environmental and genetic aspects of endocrine disruption in 80 hypospadiac and 120 age-matched control boys (56). Exposure to organochloride pesticides (OCPs) were analyzed with gas chromatography of serum. Noting that cytochrome P4501A1 (CYP1A1) and glutathione S-transferases (GSTM1 and GSTT1) are members of the xenobiotic metabolizing enzyme family that are involved in toxicant and steroid metabolism, these genes were assessed for polymorphisms. Deletion of GSTM1 and GSTT1 was significantly higher in hypospadic cases as compared to controls. These findings, however, were not found to be significant after adjusting for maternal occupational exposure. The authors conclude that irrespective of genetic predisposition, higher levels of some OCPs may be associated with increased risk of hypospadias.
Conclusions
The development of the external genitalia has been shown to be fundamentally influenced by the hormonal balance. Environmental agents that may perturb this balance have come to be known as endocrine disruptors. Historically, such findings are borne out over the span of years and decades as seen in the case of DES. The recent concerns over endocrine disruptors are especially relevant to hypospadias due to reported widespread rise in incidence of this malformation. In this review of the recent literature, a common theme was the potential association of hypospadias as a subset of the larger TDS. The majority of studies have utilized an epidemiologic approach to assess associations between potential exposures and hypospadias. Basic science studies have also been prominent, using animal and in vitro models to understand the complex pathophysiology of hypospadias. Clinical studies remain sparse. Despite the findings from these studies, there has not been definitive evidence affirming that endocrine disruptors cause hypospadias. Evidence is limited, and cause-effect relationships are difficult to establish given current evidence. However, data do indicate that there are factors beyond the endocrine disruptors themselves. The genetic pathways related to metabolism of endocrine disruptors, developmental steps, and sensitivity to these agents appears to play a role as well. Endocrine disruption may be one of the many critical factors in aberrant development of external genitalia that manifests as hypospadias.
Acknowledgements
Funding: Supported by NIH grant DK058105 Hypospadias Differentiation and Endocrine Disrupters.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Institute of Health, National Institute of Environmental Health Sciences (NIEHS). Endocrine Disruptors. Available online: http://www.niehs.nih.gov/health/topics/agents/endocrine/
- Report on Carcinogens (12th Ed). DIANE Publishing; 2011. Available online: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/diethylstilbestrol.pdf
- Bern HA, Blair P, Brasseur S, et al. Statement from the work session on chemically-induced alterations in sexual development: the wildlife/human connection. In: Clement C, Colborn T. eds. Chemically-induced alterations in sexual and functional development-- the wildlife/human connection. Princeton: Princeton Scientific Pub. Co, 1992;1-8.
- EDSP Chronology. Available online: http://www.epa.gov/endo/pubs/edspoverview/chronology.htm
- EDSP Background. Available online: http://www.epa.gov/endo/pubs/edspoverview/background.htm
- Nordenvall AS, Frisén L, Nordenström A, et al. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol 2014;191:783-9. [PubMed]
- Czeizel A. Increasing trends in congenital malformations of male external genitalia. Lancet 1985;1:462-3. [PubMed]
- Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics 1997;100:831-4. [PubMed]
- Wein AJ, Kavoussi LR, Novick AC, et al. eds. Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set, 10e. Bergen: Saunders, 2011.
- Fisch H, Lambert SM, Hensle TW, et al. Hypospadias rates in New York State are not increasing. J Urol 2009;181:2291-4. [PubMed]
- Carmichael SL, Shaw GM, Nelson V, et al. Hypospadias in California: trends and descriptive epidemiology. Epidemiology 2003;14:701-6. [PubMed]
- Porter MP, Faizan MK, Grady RW, et al. Hypospadias in Washington State: maternal risk factors and prevalence trends. Pediatrics 2005;115:e495-9. [PubMed]
- Yucel S, Cavalcanti AG, Desouza A, et al. The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. BJU Int 2003;92:1016-21. [PubMed]
- Kim KS, Torres CR Jr, Yucel S, et al. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res 2004;94:267-75. [PubMed]
- Buckley J, Willingham E, Agras K, et al. Embryonic exposure to the fungicide vinclozolin causes virilization of females and alteration of progesterone receptor expression in vivo: an experimental study in mice. Environ Health 2006;5:4. [PubMed]
- Willingham E, Agras K, Vilela M, et al. Loratadine exerts estrogen-like effects and disrupts penile development in the mouse. J Urol 2006;175:723-6. [PubMed]
- Agras K, Willingham E, Liu B, et al. Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. J Urol 2006;176:1883-8. [PubMed]
- Agras K, Shiroyanagi Y, Baskin LS. Progesterone receptors in the developing genital tubercle: implications for the endocrine disruptor hypothesis as the etiology of hypospadias. J Urol 2007;178:722-7. [PubMed]
- Agras K, Willingham E, Shiroyanagi Y, et al. Estrogen receptor-alpha and beta are differentially distributed, expressed and activated in the fetal genital tubercle. J Urol 2007;177:2386-92. [PubMed]
- Hsieh MH, Grantham EC, Liu B, et al. In utero exposure to benzophenone-2 causes hypospadias through an estrogen receptor dependent mechanism. J Urol 2007;178:1637-42. [PubMed]
- Vilela ML, Willingham E, Buckley J, et al. Endocrine disruptors and hypospadias: role of genistein and the fungicide vinclozolin. Urology 2007;70:618-21. [PubMed]
- Yong W, Yang Z, Periyasamy S, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem 2007;282:5026-36. [PubMed]
- Zhang LF, Qin C, Wei YF, et al. Differential expression of the Wnt/β-catenin pathway in the genital tubercle (GT) of fetal male rat following maternal exposure to di-n-butyl phthalate (DBP). Syst Biol Reprod Med 2011;57:244-50. [PubMed]
- Schneider S, Kaufmann W, Strauss V, et al. Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regul Toxicol Pharmacol 2011;59:91-100. [PubMed]
- Ross AE, Marchionni L, Phillips TM, et al. Molecular effects of genistein on male urethral development. J Urol 2011;185:1894-8. [PubMed]
- Ma LM, Wang Z, Wang H, et al. Estrogen effects on fetal penile and urethral development in organotypic mouse genital tubercle culture. J Urol 2009;182:2511-7. [PubMed]
- Qin XY, Sone H, Kojima Y, et al. Individual variation of the genetic response to bisphenol a in human foreskin fibroblast cells derived from cryptorchidism and hypospadias patients. PLoS One 2012;7:e52756. [PubMed]
- Qin XY, Kojima Y, Mizuno K, et al. Association of variants in genes involved in environmental chemical metabolism and risk of cryptorchidism and hypospadias. J Hum Genet 2012;57:434-41. [PubMed]
- Toppari J, Virtanen HE, Main KM, et al. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol 2010;88:910-9. [PubMed]
- Main KM, Skakkebaek NE, Virtanen HE, et al. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 2010;24:279-89. [PubMed]
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009;71:459-65. [PubMed]
- Qiao L, Rodriguez E Jr, Weiss DA, et al. Expression of estrogen receptor alpha and beta is decreased in hypospadias. J Urol 2012;187:1427-33. [PubMed]
- Padilla-Banks E, Jefferson WN, Myers PH, et al. Neonatal phytoestrogen exposure causes hypospadias in female mice. Mol Reprod Dev 2012;79:3. [PubMed]
- Gray LE Jr, Barlow NJ, Howdeshell KL, et al. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci 2009;110:411-25. [PubMed]
- Rider CV, Furr J, Wilson VS, et al. A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl 2008;31:249-62. [PubMed]
- Gray LE Jr, Ostby J, Furr J, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 2000;58:350-65. [PubMed]
- Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005;113:1056-61. [PubMed]
- Do RP, Stahlhut RW, Ponzi D, et al. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol 2012;34:614-21. [PubMed]
- Liu C, Xu X, Huo X. Anogenital distance and its application in environmental health research. Environ Sci Pollut Res Int 2014;21:5457-64. [PubMed]
- Hsieh MH, Breyer BN, Eisenberg ML, et al. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep 2008;9:137-42. [PubMed]
- Hsieh MH, Eisenberg ML, Hittelman AB, et al. Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum Reprod 2012;27:1577-80. [PubMed]
- Thankamony A, Lek N, Carroll D, et al. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect 2014;122:207-11. [PubMed]
- Choi H, Kim J, Im Y, et al. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 2012;47:2173-9.
- Meijer L, Martijn A, Melessen J, et al. Influence of prenatal organohalogen levels on infant male sexual development: sex hormone levels, testes volume and penile length. Hum Reprod 2012;27:867-72. [PubMed]
- Carmichael SL, Yang W, Roberts EM, et al. Hypospadias and residential proximity to pesticide applications. Pediatrics 2013;132:e1216-26. [PubMed]
- Agopian AJ, Lupo PJ, Canfield MA, et al. Case-control study of maternal residential atrazine exposure and male genital malformations. Am J Med Genet A 2013;161A:977-82. [PubMed]
- Rignell-Hydbom A, Lindh CH, Dillner J, et al. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One 2012;7:e44767. [PubMed]
- El Kholy M, Hamza RT, Saleh M, et al. Penile length and genital anomalies in Egyptian male newborns: epidemiology and influence of endocrine disruptors. J Pediatr Endocrinol Metab 2013;26:509-13. [PubMed]
- Gaspari L, Sampaio DR, Paris F, et al. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int J Androl 2012;35:253-64. [PubMed]
- Gaspari L, Paris F, Jandel C, et al. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Hum Reprod 2011;26:3155-62. [PubMed]
- Giordano F, Abballe A, De Felip E, et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol 2010;88:241-50. [PubMed]
- Morales-Suárez-Varela MM, Toft GV, Jensen MS, et al. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish National Birth Cohort study. Environ Health 2011;10:3. [PubMed]
- Nassar N, Abeywardana P, Barker A, et al. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med 2010;67:585-9. [PubMed]
- Kalfa N, Paris F, Soyer-Gobillard MO, et al. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril 2011;95:2574-7. [PubMed]
- Small CM, DeCaro JJ, Terrell ML, et al. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ Health Perspect 2009;117:1175-9. [PubMed]
- Shekharyadav C, Bajpai M, Kumar V, et al. Polymorphism in CYP1A1, GSTMI, GSTT1 genes and organochlorine pesticides in the etiology of hypospadias. Hum Exp Toxicol 2011;30:1464-74. [PubMed]


