My indications for treatment of the adolescent varicocele (and why?)
Introduction
Varicocele occurs in approximately 15% of the adolescent and adult male population and as many as 20% will eventually be identified with an infertility problem, most often around the time of trying to father a child. One should not make light of the high association of infertility with varicocele as it relates to millions of adult men. If informed of this strong association, I wonder how many parents would want to take the chance of future infertility in their teenage son especially if they knew that the incidence of an infertility problem is one in five and the alternative, varicocelectomy, is associated with a high success and low complication rate. Yet if we exposed all adolescent males with a varicocele to surgery, 80% would be undergoing an unnecessary operation and the cost of care would end up stifling our economy (1). The better option is to determine who with a varicocele would be most likely to develop infertility in the future so that treatment could be provided before a problem manifested. That is not so easy to accomplish as there are not enough published studies in which adolescents with a varicocele have been followed into adulthood as regards to later development of abnormal semen analyses or difficulties fathering a child nor have adequate indicators been developed to identify who will have a later problem and who will benefit most from surgical correction. Just to follow males with varicoceles into adulthood and operate once a fertility problem develops does not seem to be the best route since once infertility is identified in an adult, improvement in semen parameters following surgery will only occur in two-thirds and even in less, i.e., 40%, will paternity follow a percentage that is only marginally greater than if varicocelectomy had not been done (2).
Predicting future infertility
A number of possible indicators of future infertility have been investigated both in the past and more recently and these include varicocele grade, asymmetric testicular growth, total testicular volume (TTV), and the Doppler ultrasound (DUS) parameters of maximum vein diameter and peak retrograde flow (PRF).
Varicocele grade
As regards grade, it is rare for a child with a grade 1 left varicocele to be referred to a pediatric urologist as by definition these are not easily palpable and require a Valsalva to be detected or confirmed. In general the varicoceles that are referred to us are those grade 2 varicoceles that are more easily palpable and of course grade 3 varicoceles as these are visible through the skin from a distance.
Grade however, has not proven to be a reliable indicator of future asymmetry. For example, Diamond et al. could not identify any difference in semen parameters or testicular volume differentials between grade 2 and 3 varicoceles (3). On the other hand, Zampieri et al. identified greater ipsilateral hypotrophy amongst grade 3 varicoceles as compared to grade 2 varicoceles and our data identified greater asymmetry amongst boys with a grade 3 varicocele as compared to those with a grade 2 varicocele (4,5). And while Mori et al. were not able to identify any difference in testicular volume or sperm integrity between grade 2 and 3 varicoceles in adolescents, they did find that the total number of progressively motile sperm in grade 3 varicoceles to be very close to the World Health Organization’s (WHO) cut-off for normal (6). Adult studies, however have shown that grade and size of the varicocele are associated with ipsilateral hypotrophy and abnormal semen parameters (2,7).
Percent asymmetry
When it was realized that adult males with a left varicocele and infertility were likely to have a left testicle that was smaller than the right, Kass and Belman and subsequently other pediatric urologists began to take notice and with this information in hand began to focus on differential in volume between each side as an indication for varicocelectomy (7,8). Subsequently the degree of hypotrophy translated into what has been referred to as “testicular asymmetry”. Percent asymmetry is measured using the following formula:
The volume of each testicle is determined by the formula of length × width × depth and combining it with either the 0.71 or 0.52 coefficient for an ellipse. Most observers use the former and recently it has been proven by Sakamoto et al. that they have been correct in using the former (9). This group determined the actual volume of the testicles that were excised in prostate cancer patients with water displacement analysis and compared these volumes with preoperative volumes determined by ultrasound using both the 0.71 and 0.52 coefficients and with volumes obtained using the Prader orchidometer. The 0.71 coefficient overestimated the testicular volume by 7.2%, while the 0.52 coefficient underestimated testicular volume by 21.3%. The three individual linear measurements to be inserted into the volume formula can be obtained with calipers or more often with ultrasound with most observers seeming to consider ultrasound more reliable. Others have favored using Takihara elliptical rings or a Prader orchidometer as a more practical and less expensive means of determining volume. With the Prader orchidometer, Sakamoto et al. found those volumes obtained preoperatively were 81.7% larger than the water displacement volumes (9).
Based on our own experience, we have found the rings to yield approximately a 15% to 20% larger volume than the volume determined by ultrasound although when these volumes are plugged into the % asymmetry equation there is much less of a difference. In fact, Shiraishi et al. found that the Takihara orchidometer overestimated testicular volume as compared to ultrasound, not water displacement, by 28.2% and the difference was even greater when used for small testicles (10). At the same time, one must keep in mind that measurements obtained by ultrasound can be quite variable depending upon who is obtaining the measurements. We favor using both the Takihara rings and ultrasound when determining % asymmetry just to make sure that our ultrasound measurements are not erroneous. After all, in a cubed formula, a small error in any of the linear measurements can yield a dramatic difference in % asymmetry. Alternatives to the term “% asymmetry” have included testicular volume differential and atrophy index with all meaning the same thing (11,12).
Using absolute numbers such as a 2 or 3 cc difference in volume between the two testicles as an indicator for surgery is of little value as 3 ccs would represent a much larger % asymmetry in boys at a Tanner 1 and 2 stage of development when testes are small than in Tanner 4 and 5 boys when testes are considerably larger. As regards % asymmetry various percentages have been suggested, almost arbitrarily, as cut off values to indicate a possible future problem and these include 10%, 15% and 20%.
Semen parameters
In 1991, Haans et al. found decreased total sperm counts in 17 to 20 years old with a left varicocele and ipsilateral hypotrophy thus building the case for ipsilateral hypotrophy as an indication for adolescent varicocelectomy (13). Five years later, Paduch and Niedzielski identified statistically significant differences in 17 to 19 years old with and without a varicocele in terms of motility, number of immotile sperm, vitality, frequency of abnormal sperm morphology and number of sperm with a tapered head but not as regards semen volume or sperm density (11). There also were significant differences between the two groups in ipsilateral testicular hypotrophy, % asymmetry, vein diameters and retrograde venous flow velocity. In 2002, Cayan et al. demonstrated that varicocelectomy could correct abnormal semen parameters in 15 to 19 years old with a left varicocele, ipsilateral hypotrophy and abnormal pre-varicocelectomy semen parameters, even when the asymmetry itself did not correct (14). The mean sperm concentration in those who underwent varicocelectomy rose from 17.9 million/mL preoperatively to 59.9 million/mL postoperatively (normal >20 million/mL, by WHO criteria).
In a landmark paper by Diamond et al., a strong relationship was identified between abnormal semen parameters, particularly total motile sperm count (TMC), and % asymmetry in Tanner 5 boys with a left varicocele (3). For example, by WHO criteria a TMC of <20 million is considered abnormal. When they looked at the incidence of a TMC of <20 million amongst various degrees of asymmetry, they found it present in 15% of Tanner 5 teenagers with <10% asymmetry, 33% with 10% to 20% asymmetry, and in as many as 67% with >20% asymmetry group. When they instead looked at a TMC of <10 million, an even more abnormal finding, none were identified amongst those with <10% asymmetry, but at 10% to 20% asymmetry, 11% were identified and when the asymmetry was >20% the incidence grew to 59%. Finally, the pediatric urology community had a study that validated that increased testicular asymmetry was associated with decreased fertility or at least decreased TMCs and that a 10% asymmetry cut-off was valid to cause concern and a 20% asymmetry cut-off enough to warrant surgery.
In another study, Moursy et al. identified 53 adolescents with >20% asymmetry at the time of surgery and in none of the 53 had a preoperative semen analysis been performed (15). However, on postoperative semen analysis that had been obtained in all, all were found to have normal semen parameters (15). This is a very significant study when taking into account Diamond et al.’s 2007 landmark study. Based on Diamond et al.’s findings, had these boys with >20% asymmetry not had surgery and instead were screened at a Tanner 5 stage of development, the stage at which the postoperative semen analyses were performed by Moursy et al., the majority of these boys would instead have ended up with abnormal semen parameters (3,15).
In still another study reinforcing the significance of >20% asymmetry in Tanner 5 boys was one by Keene et al., although one with very few patients but one in which 5 of 5 teenagers at a mean age of 15.6 years were found to have a median sperm concentration of 3 million/mL, well below the WHO cut-off of 20 million/mL for normal with a range of 0.7 to 41 million/mL (16). In comparison in a similar aged group without asymmetry (unfortunately their cut-off for defining symmetry was not included) the mean sperm concentration was 26 million/mL with a range of 8.1 to 91 million/mL. While this study reinforces the significance of a 20% cut-off, it also identified that many patients who fell into their symmetry category fell below the 20 million sperm/mL cut-off for normal. Still we need further studies to reassure us that the WHO cut-offs for normal in adults also apply to Tanner 5 stage teenagers.
Total testicular volume (TTV)
In 1996, Paduch and Niedzielski found a significant direct linear relationship between TTV and sperm concentration in 17 to 19 years old boys with a varicocele (11). Christman et al. from Children’s Hospital of Philadelphia (CHOP) revisited TTV 18 years later and found it to be predictive of TMC but could not corroborate Diamond et al.’s findings regarding a strong association between % asymmetry and TMC (3,14). However, in a subsequent study, while this same investigating group again found TTV to be more important than asymmetry now noted that when severe asymmetry was associated with low TTV, TMC was at its lowest (17).
What’s happening on the right side?
If the CHOP studies are verified in the future by other groups, i.e., that TTV is more important than % asymmetry as an indicator of abnormal semen parameters, then we must consider why that would be the case (12,17). First of all we know that in at least four series of left varicoceles, one in adults, another in 17 to 20 years old, still another in 17 to 19 years old and finally one in 9 to 19 years old, right testicular volume was found to be smaller than in normal controls who did not have a varicocele (7,11,13,18). Does this mean that in some the growth of the right testicle has been limited by the presence of the left varicocele or alternatively that unidentified right retrograde venous flow or even an overlooked small but palpable right varicocele was inhibiting right testicular growth? We are of the school believing that many palpable, although small, right varicoceles are never detected in adolescents with left varicoceles. After all, the average incidence of bilateral varicocelectomy in adult series is 39% but in adolescent series it is only 5% (5). As regards our own experience, 22% of our cases have been done bilaterally, a percentage very comparable to adult series. However, in our last 100 varicocelectomies our incidence of doing a bilateral repair has fallen to 10% out of our impression that the very small right varicocele with a low PRF is not as important to repair as in the adult in whom the effects of bilateral disease has already taken its toll.
We have identified right retrograde flow on DUS in 40% (i.e., 204 of 506) of adolescents with a left varicocele; subclinical in 89 (18%), grade 1 in 51 (10%), grade 2 in 63 (12%) and grade 3 in 1 (0.2%) (5). These are numbers that correlate quite well with adult series but far different from most other adolescent series and numbers we believe are creditable as there should be no great difference in the incidence of right varicoceles amongst adolescents and adults. We have found that both subclinical and palpable varicoceles influence both the size of the right testicle, thus influencing % asymmetry determinations and therefore, which left varicoceles ultimately get recommended for surgical correction. For those with right varicoceles, TTV determinations would be especially of value in protecting decision making in patients who have an overlooked right varicocele. In patients with bilaterally palpable varicoceles, increasing left grade is associated with greater asymmetry (P=0.02) and increasing right grade with less asymmetry (P=0.01). When we looked at TTV in 140 Tanner 5 boys, we found that a lower TTV was more likely to be associated with a higher grade left varicocele and a greater chance of a palpable right varicocele being present (19).
Hemodynamic approach using DUS parameters
DUS can be used to obtain specific objective measurements aside from % asymmetry and TTV including such parameters as PRF and mean vein diameter, in addition to TTV, parameters that can be helpful in deciding who should undergo varicocelectomy. In Paduch and Niedzielski’s 1996 study on 17 to 19 years old with grade 2 and 3 varicoceles, they reported poorer semen quality in conjunction with higher PRFs and greater asymmetry (11). In three separate series, we have found that a combination of 20% asymmetry or greater and a PRF of 38 cm/s or greater to be strongly associated with persistent or worsening future asymmetry (20-22). We call this the “20/38 harbinger” and in the most recent account found that it can be extended to those with 15% asymmetry as well (22). Interestingly, a similar PRF, i.e., 40 cm/s, had already been identified by Gitlin and McCullough to be a cut-off above which adults with left varicoceles were found to have a significantly higher incidence of abnormal semen parameters (23).
Since many pediatric urologists are already employing ultrasound for determining volume measurements in their follow-up of adolescent varicoceles, obtaining these added measurements we feel should be included because of their importance for decision making even though it will have some impact on the cost of the study (1). Ultrasound technicians already are quite experienced in obtaining Doppler measurements in vessels throughout the body and additionally can be easily trained to substantiate that an adequate Valsalva is obtained in the supine position when obtaining PRF in the veins of a varicocele. We have found PRF values to have a better correlation with asymmetry than maximum vein diameter; the latter we also obtain in the supine position but without Valsalva.
Some patients will have a spike-like, short surge of flow of low amplitude at the beginning of a Valsalva. This spike represents either a very small varicocele or a slight delay in closure of an imperfect internal spermatic vein valve that allows momentary retrograde flow. In a true varicocele the flow curve tends to be sustained throughout the entire Valsalva (Figure 1). Unsustained retrograde venous flow during a Valsalva and low amplitude sustained flow do not warrant surgical correction.
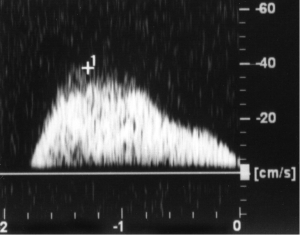
We do not go out of our way to identify the deferential vein as some have recommended (24,25). If we did we believe it would be difficult to locate but more importantly, we feel that deferential vein reflux plays little, if any, role in the development of a varicocele or a recurrence (26,27) (see section below on “Choice of varicocelectomy”). I would be interested to see reports on PRF values of deferential venous reflux from those authors who feel they are able to identify internal iliac vein reflux into the deferential vein. If these PRFs were high, one might then take deferential reflux more seriously. However, these authors need to take into account Franco et al.’s inability to identify deferential reflux in 54 patients with primary and 19 with recurrent varicocele (28).
Adolescent varicocele, a progressive disease?
Progressive disease is not unusual in patients with varicoceles during both adulthood and adolescence. Lipshultz and Corriere demonstrated progressively worse semen parameters in men with varicoceles and ipsilateral hypotrophy, and Cheval and Purcell demonstrated that in those men with hypotrophy the hypotrophy often worsens with time (2,7). Kozakowski et al. found that mean PRF of boys with a left varicocele who develop worsening asymmetry or new onset asymmetry also can occur during adolescence particularly in boys with a mean PRF of 38 cm/s (20). For example, they showed that new asymmetry could develop in adolescents with initial symmetry and in these boys the mean PRF of the group was 38 cm/s. And when worse asymmetry developed in boys already with asymmetry, the mean PRF again was 38 cm/s, curiously the same PRF that is associated with the “20/38 harbinger” discussed above (20). Both Gorelick and Goldstein and Witt and Lipshultz showed that varicocele is a progressive disease by comparing the higher incidence of varicocele in men with secondary infertility (i.e., previously had fathered a child and subsequently could not) as compared to men with primary infertility (i.e., never father a child) (29,30). For example, in the former report 35% of men with primary infertility had a varicocele while in those with secondary infertility, i.e., the older group, had an 82% incidence of varicocele (29). The corresponding percentages in the Witt and Lipshultz group were 50% and 69%, a bit less provocative but still significant (30). That there was a dramatic increase in infertility in adults with secondary paternity and obviously in those who were older supports the progressive nature of the disease. It therefore, seems preferable to do a varicocelectomy when younger than when older when appropriate indications exist, especially since only two-thirds of adults will have improvement in sperm parameters following varicocelectomy and in teenagers normalization of abnormal parameters appears to be almost universal following varicocelectomy and certainly better than that seen in adult males later diagnosed with infertility. Not only does there seem to be a better responsiveness of the testicle to varicocelectomy when younger but as well the chance for paternity following varicocelectomy likely is better when varicocelectomy is performed earlier. The concern over future fertility is so great that Keene et al. have encouraged sperm banking for postpubertal young men with varicoceles (16). Finally, Chen and Chen demonstrated progressively abnormal semen parameters in men with varicoceles, particularly in those with higher PRFs (31).
The progressive effect of a left varicocele on the right side has been suggested by Kass et al. when they discovered that right testicles in the presence of a left varicocele were smaller than in boys without a varicocele but the difference in right testicular volume as compared to boys who did not have a varicocele did not become statistically significant until a Tanner 4 or 5 stage of development was reached (18).
Indications for varicocelectomy
If we are to do an adolescent varicocelectomy it must be done for the correct reasons. While we feel strongly that % asymmetry is a useful tool to help identify who should have surgery, we do recognize that some adolescents will have catch-up growth overtime without surgery, in some studies more often than in others. For example, Kolon et al. found that 74% of adolescents with >15% asymmetry will have catch-up growth without surgery to less than 15% asymmetry over a median follow-up of 39 months (32). We on the other hand found that only 33% of those with >15% asymmetry had catch-up growth to less than 15% but with a shorter follow-up, i.e., 21 months (21). The point is that catch-up growth does occur without surgery but in our experience much less often than the Kolon et al. report suggests (21,32). It therefore is important when using asymmetry as a guideline for determining who should be followed and who should undergo surgery, that we have predictors of who will have catch-up growth and who will have persistent or greater asymmetry on follow-up, and as you see above that is what we have been fairly successful at developing (22).
It is important to maintain an awareness of Diamond et al.’s aforementioned finding, i.e., 59% of Tanner 5 boys with >20% asymmetry have a TMC of less than 10 million, a value that is clearly abnormal (3). It is preferable to identify these individuals before they reach a Tanner 5 stage of development and before they are found to have abnormal semen parameters. It is preferable to identify those who are likely to have persistent or worsening asymmetry in the future. Therefore, we rely heavily on the “20/38 harbinger” and more recently even a “15/38” cut-off when deciding upon surgery (22). If someone falls within these guidelines early in puberty, we will not hesitate to recommend surgery rather than wait to see if catch-up growth will occur with watchful waiting or await the patient’s reaching a Tanner 5 stage of development in order to obtain a semen analysis. I prefer early surgery for appropriate indications and avoid the need to reverse abnormal semen parameters once they have developed. We rely more on semen analyses for those with border-line asymmetry and border-line PRF values and for whom surgery has not yet been advised. We have always been concerned about the adolescent who has very elevated PRFs but in whom both testes are symmetrically small and although his testes are small he does not fit into the usual guidelines for surgery. As a result, and perhaps more on the basis of common sense, we have of recent years recommended surgery for such adolescents. Now I look forward to CHOP or perhaps some other institution to establish normal TTV values for each Tanner stage and to determine what TTV cut-off value might prove useful in guiding treatment.
On the basis of the CHOP findings as regards the importance of TTV, TTV should be put into the decision making process to help in determining the need for surgery and perhaps even better, some yet to be determined combination of TTV, % asymmetry and PRF might become the best indicator (17,19,22). While TTV may turn out to be very valuable in predicting abnormal semen parameters, it is doubtful that with so many studies proving a relationship between abnormal serum parameters and asymmetry that the usefulness of asymmetry will fall out of use any time soon. Pain, although an unusual presenting symptom in adolescence, remains an indication for surgery.
Some may object to advising surgery on the basis of one DUS study that meets the 15/38 harbinger guidelines. They might say: “What is lost by waiting another year even if we fall behind as regards a possible decline in semen parameters” and then might add: “When varicocelectomy is done in the older teenage boy, abnormal semen parameters usually normalize anyway.” (14,15,33). In addition, andrologists are now excellent at achieving pregnancy in couples with the husband having low sperm and motility values. Yes the andrologists are great at what they do but at an extremely high financial cost that is preceded by an extremely high emotional cost. And as stated above why wait for an abnormal spermiogram to develop and then have to reverse it. Who is to say that once abnormal parameters are reversed how long they then will stay normal in a testicle that already has incurred damage (1).
Choice of varicocelectomy
The two methods of varicocelectomy that have gained the most popularity amongst pediatric urologists in the United States are the Palomo repair with high inguinal en block ligation and the laparoscopic approach (34). Radiographic embolization is seldomly used for a primary repair because of the difficulty gaining access to the ostium of the left testicular vein and a relatively high recurrence rate. Antegrade sclerotherapy is widely used in Europe and yields a high success rate with little risk of hydrocele formation (35,36). Pediatric urologists are less likely than andrologists to use the microscopic approach (only 1% of adolescent varicocelectomies in one recent national survey) because of limited experience with it and the fear of the quite rare instance of post-varicocelectomy ipsilateral testicular atrophy (34,37). The Palomo repair has an undeniable high incidence of postoperative hydroceles, as high as 30%, but with the majority not requiring a hydrocelectomy (38-40). Wong et al. have effectively lessened the incidence of hydroceles with the Palomo repair by saving the lymphatics with the use of the operative microscope (41).
Most pediatric urologists in the United States prefer the laparoscopic approach (54%) in part because of its high success rate but also because it facilitates preservation of lymphatics which in turn keeps the incidence of postoperative hydroceles low (27,34,42,43). While saving the artery can be accomplished laparoscopically, it is associated with a higher recurrence rate (27,33). Zampieri et al. found that saving the artery led to better postoperative semen parameters than when the artery was ligated (33). However, the mean results for sperm concentration, % normal morphology, volume, and vitality were in the normal range, i.e., >20 million/mL, >30%, >2 cc, and >70%, respectively, for both the artery sparing and artery ligating groups or in other words artery sparing gave “more normal” results. On the other hand, mean progressive motility values for both groups fell below the WHO 50% cut-off for normal, being 39% in the artery ligation group and 47% in the artery sparing group. Unfortunately because the results were published in terms of mean and range, we do not know how many from each group had abnormal parameters and because surgery was done between 12 and 16 years of age, no preoperative semen analyses were obtained. The postoperative spermiograms were obtained after each patient’s 18th birthday (33). As regards postoperative recurrences, 6 of the 63 (10%) boys in their series who underwent artery sparing laparoscopic varicocelectomy developed a recurrence, while none of the 59 patients in whom the artery was ligated developed a recurrence. In our own series, we also had an increased incidence of recurrence with artery sparing, i.e., 10% in our artery sparing group vs. 4% in the non-artery sparing group (27). As regards catch-up growth, there was no difference between the two groups.
In those patients with a recurrence following a laparoscopic varicocelectomy, we have had a 100% success rate with redo surgery using a high inguinal exposure with isolation of the cord just caudad to its junction with the deferential vein (26). In only one patient did we identify a dilated deferential vein and in that case we ligated it. In all the redos, large veins were identified within the cord and in all cases they were in continuity with large veins that could be seen cephalad to where the vas deferens joins the cord or in other words just caudad to where the internal spermatic veins were ligated at the original laparoscopic procedure. In addition, no perforators were identified in the floor of the inguinal canal and in no case was the testicle mobilized into the inguinal wound in order to search for scrotal collaterals. In only one case was a dilated external spermatic vein identified and it was ligated. We have no way of knowing if this dilated external spermatic vein or the above mentioned deferential vein had reflux nor how these two patients would have fared had these vessels not been ligated. In fact, not all dilated veins have reflux (28). When one considers that we have done over 500 laparoscopic varicocelectomies, with a recurrence rate of 4% to 5% and that all recurrences were successfully repaired this translates into an overall 100% success rate although some patients needed two surgeries for cure (26,27). Considering that the laparoscopic procedure does not allow visualization of the inguinal canal to identify collaterals, that only one dilated deferential vein and one dilated external spermatic vein were ligated at the time of redo surgery and no testicle was mobilized out of the scrotum, we feel that the role of collaterals caudad to the internal ring has little if any relationship to the etiology of a varicocele or its recurrence.
For reasons similar to our not investigating for deferential reflux on DUS (see section above “Hemodynamic approach using DUS parameters”), we also do not use the Coolsaet DUS classification in determining what our surgical approach should be as Cimador et al. recommend using a modified Coolsaet DUS grading system for varicocele, i.e., laparoscopic approach for a Coolsaet grade 1 varicocele which represents a varicocele with only internal spermatic vein reflux as opposed to a subinguinal microscopic approach for a Coolsaet grade 3 varicocele in which reflux has been demonstrated in both the internal spermatic and deferential veins (24,25,44). A modified Coolsaet grade 2 varicocele is one that only has deferential vein reflux and no internal spermatic vein reflux and according to Cimador et al.’s study a rare finding.
We also do not go along with Dudai et al.’s recommendation that the external (also referred to as cremasteric vein) and internal spermatic veins should be ligated at varicocelectomy since, as we stated above, in only one of over 500 cases did we ligate an external spermatic vein and in addition, Cimador et al. did not find any instance of external spermatic vein reflux in 148 DUS studies (27,45,46).
As regards Goldstein et al.’s initial recommendation that the testicle be mobilized into the inguinal wound to identify scrotal vein collaterals outside the cord is now being questioned (26,47). For example, Ramasamy and Schlegel found no advantage to ligating scrotal veins at the time of subinguinal microscopic varicocelectomy in terms of postoperative semen quality or pregnancy rats nor in an increased incidence of recurrence (48). What’s more, Franco et al. on venography in 73 patients found no evidence of venous reflux into any scrotal, deferential, or internal spermatic vein, findings that corroborate our observations regarding the insignificance of collaterals below the internal inguinal ring (26,28). They did find instances of dilated external spermatic and deferential veins but without reflux and credited this finding to venous overflow due to insufficiency of the internal spermatic vein or possibly partial obstruction of the left internal iliac vein. They therefore, question as do we, the rationale for ligating these extrafunicular veins as they refer to them. If the deferential, external spermatic, scrotal and perforating veins are not the cause of a recurrence, we then wonder what vein or veins are the culprit?
Adolescent varicocelectomy has been shown to lead to postoperative catch-up growth in 70% to 80% of boys with preoperative asymmetry (8,14,40,49). In our own experience, the incidence of catch-up growth following non-lymphatic sparing laparoscopic varicocelectomy was 71%, after lymphatic sparing 80%, and following lymphatic and artery sparing 83% (27). Some have suggested that ligation of the lymphatics accounts for most of the post-operative catch-up growth that occurs. Our data does not support this hypothesis as there was no significant difference in catch-up growth between those in whom lymphatics were ligated or spared (27,49).
Paternity outcomes
We are only aware of two studies that have looked at paternity as an outcome from adolescent varicocelectomy (50,51). In one of the two which was our own report, we reported on the results of a questionnaire that was sent to 50 Hasidic/ultraorthodox Jewish families in whom birth prevention is forbidden, marriage occurs at an early age, and in whom the index cases were now over 22 years of age and had had a varicocelectomy as a teenager (50). The majority had undergone surgery for the presence of >10% asymmetry. Of 50 who received a questionnaire, 43 responded and 18 had already been married. In all 18 marriages, conception had been achieved within the first year of their marriage (38). In the second paper, Pajovic and Radojevic reported a 75% pregnancy rate in men who had undergone varicocelectomy between ages 15 to 19 for abnormal semen parameters (51). The authors felt, and rightly so, the incidence of paternity would rise for the group as more men became interested in fathering a child with the passage of time. Meanwhile that paternity following varicocelectomy in infertile males only leads to marginal improvement in the incidence of paternity and the high incidence of paternity in the above two studies leads us to believe that the ability to respond to varicocelectomy in terms of improved paternity decreases with age—another example of progressive injury.
Lastly, I will respond to Kogan and Hollowell’s question: “Does catch-up growth lead to better adult semen analyses or better fertility?” (1). The answer is not fully in but the normal semen analyses found after teenage varicocelectomy in studies by Cayan et al., Zampieri et al., and Moursy et al. and the high incidence of paternity in the studies performed by Salzhauer et al. and Pajovic and Radojevic following adolescent and teenage varicocelectomy supports our intuitive belief that adolescent varicocelectomy that is performed with the intent of correcting testicular asymmetry and/or low TTV offers a better outcome for these adolescents, one with better semen parameters, even in some who do not achieve postvaricocelectomy catch-up growth, and a better opportunity as an adult for paternity (14,15,33,50,51).
Conclusions
There is a strong relationship between ipsilateral hypotrophy and abnormal semen parameters in both adults and teenage boys with a left varicocele. Both asymmetry and semen parameters can worsen with time, lending evidence to the progressive nature of the condition. While catch-up growth can occur without surgery, our observations have led us to believe that catch-up growth will occur only one-third of the time over a mean follow-up of 21 months. Meanwhile, over this period of observation the right testicle might not grow as well as in boys without a varicocele, especially if there is a right varicocele, a finding that occurs much more often than most pediatric urologists are willing to accept. Smaller right testicles, whether smaller for some unknown relationship with the left varicocele or because of right retrograde venous flow can lead to less asymmetry and perhaps as a result less left varicocelectomies for some teenagers who might benefit from the operation. There is an extremely strong relationship between high PRF and persistent and worsening asymmetry and presumably increased abnormal semen parameters. It is best to operate early when the indications are appropriate such as a PRF of 38 cm/s or greater, the presence of 15% or 20% asymmetry or greater, or TTV is low for a particular Tanner stage. Continuing to observe patients with the above findings is not as conservative as one might at first think. Waiting until a Tanner 5 stage or 17 or 18 years of age is reached when it is easier for a physician to request a semen analysis likely will yield abnormal semen parameters in the majority at a time that a varicocelectomy will then be required to reverse the abnormal parameters. The alternative of waiting until an infertility problem presents itself does not seem to be the best option as this is a period of life when there is less likelihood that varicocelectomy will correct abnormal semen parameters or improve the chance of fathering a child. In the next few years, TTV likely will play a role in addition to asymmetry as a marker for surgery. We await studies that will let us know what are normal testicular volumes and TTVs for each Tanner stage. Meanwhile the prophetic nature of the “20/38 harbinger” or even the “15/38 harbinger” should not be overlooked.
Acknowledgements
To Anthony Gaselberti for his passion in performing our DUS studies and the invariable dialogue that followed each study. To Jason Van Batavia, MD, who has co-authored with me many of our papers that I have referenced in this manuscript and for sustaining our registry.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kogan BA, Hollowell JG. Varicocele and value based health care. J Urol 2013;189:1626-7. [PubMed]
- Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril 1992;57:174-7. [PubMed]
- Diamond DA, Zurakowski D, Bauer SB, et al. Relationship of varicocele grade and testicular hypotrophy to semen parameters in adolescents. J Urol 2007;178:1584-8. [PubMed]
- Zampieri N, Zuin V, Corroppolo M, et al. Relationship between varicocele grade, vein reflux and testicular growth arrest. Pediatr Surg Int 2008;24:727-30. [PubMed]
- Woldu S, Nees S, Van Batavia J, et al. Physical exam and ultrasound characteristics of right varicocoeles in adolescents with left varicocoeles. Andrology 2013;1:936-42. [PubMed]
- Mori MM, Bertolla RP, Fraietta R, et al. Does varicocele grade determine extent of alteration to spermatogenesis in adolescents? Fertil Steril 2008;90:1769-73. [PubMed]
- Lipshultz LI, Corriere JN Jr. Progressive testicular atrophy in the varicocele patient. J Urol 1977;117:175-6. [PubMed]
- Kass EJ, Belman AB. Reversal of testicular growth failure by varicocele ligation. J Urol 1987;137:475-6. [PubMed]
- Sakamoto H, Saito K, Oohta M, et al. Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology 2007;69:152-7. [PubMed]
- Shiraishi K, Takihara H, Kamiryo Y, et al. Usefulness and limitation of punched-out orchidometer in testicular volume measurement. Asian J Androl 2005;7:77-80. [PubMed]
- Paduch DA, Niedzielski J. Repair versus observation in adolescent varicocele: a prospective study. J Urol 1997;158:1128-32. [PubMed]
- Christman MS, Zderic SA, Canning DA, et al. Active surveillance of the adolescent with varicocele: predicting semen outcomes from ultrasound. J Urol 2014;191:1401-6. [PubMed]
- Haans LC, Laven JS, Mali WP, et al. Testis volumes, semen quality, and hormonal patterns in adolescents with and without a varicocele. Fertil Steril 1991;56:731-6. [PubMed]
- Cayan S, Akbay E, Bozlu M, et al. The effect of varicocele repair on testicular volume in children and adolescents with varicocele. J Urol 2002;168:731-4. [PubMed]
- Moursy EE, ElDahshoury MZ, Hussein MM, et al. Dilemma of adolescent varicocele: long-term outcome in patients managed surgically and in patients managed expectantly. J Pediatr Urol 2013;9:1018-22. [PubMed]
- Keene DJ, Sajad Y, Rakoczy G, et al. Testicular volume and semen parameters in patients aged 12 to 17 years with idiopathic varicocele. J Pediatr Surg 2012;47:383-5. [PubMed]
- Kurtz MP, Rosoklija I, Johnson KL, et al. Combined correlation of total testis volume and volume differential with total motile sperm counts in adolescent varicocele. J Urol 2014;191 Supplement:e254-e255.
- Kass EJ, Stork BR, Steinert BW. Varicocele in adolescence induces left and right testicular volume loss. BJU Int 2001;87:499-501. [PubMed]
- Van Batavia JP, Finkelstein J, Tohme S, et al. Significance of total testicular volume as a prognostic factor in the adolescent varicocele. Pediatric Urology Fall Congress. Las Vegas, Nevada, 2013:September 20-22.
- Kozakowski KA, Gjertson CK, Decastro GJ, et al. Peak retrograde flow: a novel predictor of persistent, progressive and new onset asymmetry in adolescent varicocele. J Urol 2009;181:2717-22. [PubMed]
- Poon SA, Gjertson CK, Mercado MA, et al. Testicular asymmetry and adolescent varicoceles managed expectantly. J Urol 2010;183:731-4. [PubMed]
- Van Batavia JP, Badalato G, Fast A, et al. Adolescent varicocele-is the 20/38 harbinger a durable predictor of testicular asymmetry? J Urol 2013;189:1897-901. [PubMed]
- Gitlin J, McCullough A. Peak venous flow: a novel sonography parameter for evaluating patients with varicoceles. Presented at annual meeting of New York Academy of Medicine, New York, New York 2001.
- Coolsaet BL. The varicocele syndrome: venography determining the optimal level for surgical management. J Urol 1980;124:833-9. [PubMed]
- Cimador M, Di Pace MR, Peritore M, et al. The role of Doppler ultrasonography in determining the proper surgical approach to the management of varicocele in children and adolescents. BJU Int 2006;97:1291-7. [PubMed]
- Glassberg KI, Badalato GM, Poon SA, et al. Evaluation and management of the persistent/recurrent varicocele. Urology 2011;77:1194-8. [PubMed]
- Glassberg KI, Tohme S, Fast AM, et al. Laparoscopic varicocelectomy: a single surgeon's experience with over 500 cases. Pediatric Urology Fall Congress. Las Vegas, Nevada, 2013:September 20-22.
- Franco G, Iori F, de Dominicis C, et al. Challenging the role of cremasteric reflux in the pathogenesis of varicocele using a new venographic approach. J Urol 1999;161:117-21. [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [PubMed]
- Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology 1993;42:541-3. [PubMed]
- Chen SS, Chen LK. Risk factors for progressive deterioration of semen quality in patients with varicocele. Urology 2012;79:128-32. [PubMed]
- Kolon TF, Clement MR, Cartwright L, et al. Transient asynchronous testicular growth in adolescent males with a varicocele. J Urol 2008;180:1111-4. [PubMed]
- Zampieri N, Zuin V, Corroppolo M, et al. Varicocele and adolescents: semen quality after 2 different laparoscopic procedures. J Androl 2007;28:727-33. [PubMed]
- Harel M, Herbst KW, Nelson E, et al. Practice Patterns in the surgical approach for adolescent varicocelectomy. Pediatric Urology Fall Congress. Miami Beach, Florida, 2014:October 24-26.
- Fette A, Mayr J. Treatment of varicoceles in childhood and adolescence with Tauber's antegrade scrotal sclerotherapy. J Pediatr Surg 2000;35:1222-5. [PubMed]
- Mazzoni G. Adolescent varicocele: treatment by antegrade sclerotherapy. J Pediatr Surg 2001;36:1546-50. [PubMed]
- Zampieri N, Cervellione RM. Varicocele in adolescents: a 6-year longitudinal and followup observational study. J Urol 2008;180:1653-6. [PubMed]
- Kass EJ, Marcol B. Results of varicocele surgery in adolescents: a comparison of techniques. J Urol 1992;148:694-6. [PubMed]
- Misseri R, Gershbein AB, Horowitz M, et al. The adolescent varicocele. II: the incidence of hydrocele and delayed recurrent varicocele after varicocelectomy in a long-term follow-up. BJU Int 2001;87:494-8. [PubMed]
- Feber KM, Kass EJ. Varicocelectomy in adolescent boys: long-term experience with the Palomo procedure. J Urol 2008;180:1657-9. [PubMed]
- Wong J, Chan S, Pagala M, et al. Lymphatic sparing microscopic retroperitoneal varicocelectomy: a preliminary experience. J Urol 2009;182:2460-3. [PubMed]
- Kocvara R, Dvorácek J, Sedlácek J, et al. Lymphatic sparing laparoscopic varicocelectomy: a microsurgical repair. J Urol 2005;173:1751-4. [PubMed]
- Glassberg KI, Poon SA, Gjertson CK, et al. Laparoscopic lymphatic sparing varicocelectomy in adolescents. J Urol 2008;180:326-30. [PubMed]
- Cimador M, Castagnetti M, Gattuccio I, et al. The hemodynamic approach to evaluating adolescent varicocele. Nat Rev Urol 2012;9:247-57. [PubMed]
- Dudai M, Sayfan J, Mesholam J, et al. Laparoscopic simultaneous ligation of internal and external spermatic veins for varicocele. J Urol 1995;153:704-5. [PubMed]
- Cimador M, Di Pace MR, Sergio M, et al. Laparoscopic surgery of deferential reflux in pediatric and adolescent varicocele. J Laparoendosc Adv Surg Tech A 2009;19 Suppl 1:S133-6. [PubMed]
- Goldstein M, Gilbert BR, Dicker AP, et al. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol 1992;148:1808-11. [PubMed]
- Ramasamy R, Schlegel PN. Microsurgical inguinal varicocelectomy with and without testicular delivery. Urology 2006;68:1323-6. [PubMed]
- Poon SA, Kozakowski KA, Decastro GJ, et al. Adolescent varicocelectomy: postoperative catch-up growth is not secondary to lymphatic ligation. J Pediatr Urol 2009;5:37-41. [PubMed]
- Salzhauer EW, Sokol A, Glassberg KI. Paternity after adolescent varicocele repair. Pediatrics 2004;114:1631-3. [PubMed]
- Pajovic B, Radojevic N. Prospective follow up of fertility after adolescent laparoscopic varicocelectomy. Eur Rev Med Pharmacol Sci 2013;17:1060-3. [PubMed]


