Management of radiation-induced urethral strictures
Incidence of urethral strictures after radiation therapy
Prostate cancer affects approximately 30% of men and remains the most common cancer with 233,000 new cases projected in 2014 (1). Treatment options for prostate cancer include radical prostatectomy, external beam radiation (EBR), brachytherapy (BT) or a combination of both (2). According to a current report by Jarosek et al. utilizing the Surveillance Epidemiology and End Results (SEER) Medicare linked data from 1992-2007 with follow-up through 2009, 44% of patients choose EBR, 14% BT, and 12% EBR + BT for primary treatment as compared to 27% who elect radical prostatectomy (3).
Each treatment modality has a different array of side effects, which can be immediate, medium-, and long-term (2). One of the side effects of radiation therapy is the development of urethral strictures, which occur in 2% of patients undergoing EBR, 4% for BT therapy and 11% of EBR-BT combination therapy (4). Recently, urethral stricture rates of nearly 32% were reported for patients treated with high-dose BT (5) of which the bulbomembranous part of the urethra is most commonly involved (6,7).
Pathology of radiation damage
Ionizing radiation has direct effects to tissue such as DNA damage as well as indirect effects such as free radical formation within the cell. In both instances, subsequent cell apoptosis or loss of function will occur. Such tissue damage results in replacement of the native tissue with fibrocytes, which are unable to synthesize collagen (8), and also impaired vascularization of the tissue as the basement membranes of vessels are likewise affected by the radiation effects. Pelvic radiation affects both the gastrointestinal and genitourinary tracts and presentation of the above mentioned pathophysiologic processes include colonic strictures, fistulas and radiation colitis (9) as well radiation cystitis, prostatic necrosis, and urethral strictures. The latter are the result of periurethral fibrosis, atrophy, and subsequent tissue contraction. The time course for development of a urethral stricture after radiation is highly variable and accurate reports are sparse. In one report, Merrick et al. reported a median time to urethral stricture development of 26.6 months (range, 7.8-44.1 months) after BT. Risk factors for the development of radiation-induced strictures are transurethral resection of the prostate prior to radiation regardless of whether EBR or BT was used (10) as well as age, non-white race, low income and increased comorbidity status (BT use only) (11).
The tissue damage mentioned above, specifically the poor vascularity of the radiated tissue also impedes the repair of radiation-induced strictures (12). Furthermore, these pathophysiologic processes are thought to be responsible for the high recurrence rate after urethroplasty in these patients which has been reported to be as high as 30% (7,13), in comparison to the 16% recurrence rate of urethral strictures overall.
Patient presentation and diagnostic work-up
Patients that underwent radiation therapy may present with irritative or obstructive urinary symptoms. Bladder spasms in combination with radiation cystitis and/or hematuria are present in nearly 5% of patients undergoing EBR or BT and in nearly 7% of patients that were treated with EBR and BT combination therapy (3). In patients that develop a bladder outlet obstruction due to radiation-induced urethral stricture, obstructive symptoms may occur that develop gradually and progressively over months or even years (14). Diagnostic work-up is similar to that for strictures of other etiologies and grossly follows guidelines for other obstructive conditions such as BPH. The basic evaluation should include a detailed history, physical examination, labs and urine analysis and culture. Although there currently is no established questionnaire for the subjective evaluation of symptoms and quality of life related to urethral strictures regardless of etiology, promising attempts have been reported by Jackson et al. (15), which need to be validated and tailored to the radiation etiology of the stricture.
Retrograde urethogram and voiding cystourethrography are necessary to evaluate the location and extent of the stricture. Retrograde endoscopic visualization (and antegrade if suprapubic tube access is established) determines the presence and extent of radiation necrosis of the prostate which is important to recognize as the necrotic tissue should be resected before an urethroplasty is attempted. Additional imaging modalities such as computer tomography or magnet resonance imaging are rarely indicated as the diagnostic benefit is marginal except perhaps when simultaneous tumor recurrence is suspected. Ultrasound to determine the extent of spongiofibrosis, however, may be a useful addition (16,17). In cases where it is difficult to differ between predominance of irritative or obstructive symptoms pressure-flow studies may be indicated. Determination of bladder capacity is essential as men with a small bladder capacity (<200 cc) as a result of radiation damage may need counseling regarding concomitant bladder augmentation or the feasibility of a staged continence procedure such as artificial urinary sphincter placement. Of note, in patients with severe radiation damage to the bladder with resulting low capacity and poor compliance that is refractory to other therapeutic interventions a simple cystectomy and urinary diversion may be considered.
Sexual function including erectile and ejaculatory competence should be recorded prior to surgical management of urethral strictures. Baseline erectile function may be preserved in 50% of patients undergoing urethroplasty procedures after radiation therapy while ejaculatory function has not been studied in this setting (7).
Surgical therapeutic approaches
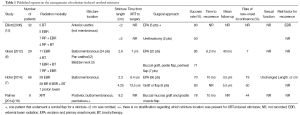
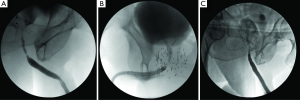
Given the unique challenges imposed by radiation therapy-induced urethral stricture several modalities have been proposed for surgical management. In general, treatment of these strictures requires consideration of the characteristics of the patient’s anatomy after radiation, viability of tissue and regenerative potential, and sphincter competence along with potential incontinence. As demonstrated in Table 1, several studies have reported on the surgical management of radiation-induced urethral strictures. The first decision that needs to be made is whether the stricture is amenable to excision and primary anastomosis (EPA), which we have found to be appropriate for the majority of radiation-induced strictures (7). EPA is desirable whenever possible as the procedure in theory is more durable in the irradiated field and avoids the use of a graft or flap. Patients with proximal bulbomembranous urethral strictures of 2-3 cm length are appropriate candidates for EPA with success rates between 70-95% (6,7,13). Risk factors for recurrence are stricture length greater than 2 cm (7) and the use of EBR instead of BT (13). Figure 1 demonstrates typical retrograde urethrograms of patients with bulbar urethral strictures after radiotherapy amenable to EPA. The major side effect of this approach can be the new-onset of urinary incontinence, which has been observed in 7-19% of patients (6,7) due to the periurethral dissection sometimes involving the membranous urethra. Yet staged placement of an AUS has been found to be successful for the treatment of urinary incontinence in men with an adequate bladder capacity following urethroplasty (7). Sexual function in these patients appears to be unchanged and rates of post-operative erectile dysfunction are consistent with previously reported rates in the post-radiation setting (19).
Full table
For more lengthy urethral strictures, substitution urethroplasty with grafts and flaps should be considered. Introduction of non-irradiated tissue in the case of buccal mucosa grafts may be superior to genital fasciocutaneous flaps secondary to the fact that the flap tissue in an irradiated patient may have also been compromised by the effects of the previous radiation exposure (20). Success rates of graft and flap urethroplasty are reportedly between 71-83% (6,7), however the numbers of patients included in the respective studies were small and the results may not be entirely representative. Another approach reserved for patients in whom extensive reconstruction is required is the combined use of buccal mucosa grafts and gracilis muscle flaps (18). Gracilis muscle flaps have been used successfully for the repair of rectourethral fistula in which viable tissue needs to be interposed between the rectum and urethra. For urethral stricture repair, gracilis muscle provides a vascular bed for ventral onlay of a buccal mucosal graft which otherwise would not be able to develop sufficient neovascularization. Palmer et al. report a success rate of this procedure of 78% in the irradiated patient (18).
Endoscopic management
Endoscopic management such as dilation followed by direct visual internal urethrotomy (DVIU) of radiation-induced strictures as first-line treatment has been proposed (21). However, these men have a high risk for recurrence, which reportedly is nearly 50% for patients who underwent BT (11). Multiple DVIU attempts should be discouraged as they make eventual reconstruction more difficult.
Excision and primary anastomosis (EPA)
An important concept is to allow for urethral rest several weeks prior to urethroplasty by exchanging an indwelling Foley catheter for a suprapubic tube to allow for tissue recovery. This has several advantages as it enables the stricture to declare its true extent before the urethroplasty procedure. Furthermore, periurethral inflammation is decreased which may impede the wound healing process following urethroplasty. Symptomatic bacteriuria should be treated prior to surgery, and asymptomatic bacteruria can be approached with 24-hour coverage with intra-venous broad-spectrum antibiotics prior to the procedure.
Patients are positioned in the dorsal lithotomy position to expose the perineum followed by covering the anus securely with a sterile towel. A vertical midline incision is made in the perineum. The bulbospongiosus muscle is encountered after division of the subcutaneous tissue and fascia of Colles and is transected to expose the urethra. This is followed by circumferential mobilization of the urethra from the penoscrotal junction to the proximal bulbar urethra using sharp dissection. On occasion the corpus cavernosum can be split to provide room to dissect and aid in the exposure of the apex of the prostate. The location of the stricture is determined intraoperatively by flexible urethroscopy and passage of a 24 French bougie à boule. The urethra is transected at the level of the stricture and inspected. Abnormal urethral mucosa and spongiofibrosis is further excised until healthy mucosa and corpus spongiosum is reached. Failure to remove all abnormal urethra will lead to stricture recurrence. Next, both ends of the urethra are widely spatulated for approximately 1 cm on both ends (26 F). The apex of the prostate should accommodate a 28 F bougie à boule.
Absorbable sutures are placed to approximate mucosa to mucosa. Care should be taken that the anastomosis is not under tension, which if it occurs, requires further mobilization of the urethra. An 18 F Foley catheter is placed prior to tying all anastomotic sutures. The bulbospongiosus muscle is approximated with 3-0 Vicryl and the fascia of Colles is closed over the muscle with the same suture. Antibiotic prophylaxis is continued for 24 hours post-operatively and the catheter is left in place for 3 weeks. Post-operative follow-up includes regular assessment of recurrence of lower urinary tract symptoms and post-void residual bladder volumes. A cystoscopy is performed if there is concern for stricture recurrence based on this assessment or if the patient experiences recurrent urinary tract infections. Of note, we do not obtain post-operative imaging studies for our patients treated with EPA.
Substitution urethroplasty
Buccal mucosa graft harvest
Graft interposition may be required if the stricture length surpasses the potential of the patient’s urethra to be mobilized sufficiently to allow for a tension free primary anastomosis. Buccal mucosa grafts have become the most commonly used graft for reconstructive surgery with excellent outcomes in the general setting (22). Among the advantages of buccal mucosal grafts is its decreased propensity for contraction, its thin lamina propria with good vascularization, lack of irradiation in this circumstance and its flexibility, which allow it to easily be adjusted according to the recipient bed shape (23). Harvest of buccal mucosa has been described extensively elsewhere (24).
The buccal mucosa graft can be applied as a ventral or dorsal onlay graft. In the bulbar urethra in which the majority of the radiation-induced strictures are located, the urethra is located dorsally in the corpus spongiosum. Applying the graft dorsally theoretically allows preservation of remaining spongiosal blood supply and neovascularization of the graft bed is facilitated by its position over the corpora cavernosa. For these reasons dorsal onlay is the preference of the authors. For substitution urethroplasty with graft, patient positioning and dissection is identical to that described above for EPA. Once the bulbar urethra is exposed and the exact location of the stricture identified the urethra is opened through a vertical incision with a scalpel. The incision should surpass the extent of the stricture proximally and distally. For a dorsal onlay graft, the bulbar urethra is mobilized to expose the urethra away from the corpora cavernosum. In our experience we suture the buccal mucosa graft into the urethrotomy with care being taken to incorporate corpora cavernosum as the graft bed. The urethral catheter is left in place for three weeks with a retrograde urethrogram performed prior to removal to assess for a leak. In ventral buccal mucosa onlay grafts vessel in growth occurs primarily from the corpus spongiosum and this technique may be considered if the tissue conditions encountered would allow for sufficient vascularization. The main advantages associated with ventral placement are easier access to the urethra, better exposure of the strictured segment thus facilitated evaluation and graft placement, and the fact that a mobilization of the bulbar urethra can be omitted (25).
Flaps and pedicled grafts
In extreme cases in which prior radiation therapy or additional subsequent failed reconstructive procedures have left extensive scar tissue with poor vascularization behind, flaps or pedicled grafts can be considered as an alternative. Often, these patients have extensive strictures (see Table 1) and unlike free grafts (such as buccal mucosa grafts) they bring their own blood supply with them thus allowing for wound healing which otherwise would not be possible in such wounds. Pedicled graft using scrotal skin, perineal skin, or penile skin have been reported and may be used. However, if such extensive repair is required, a combined repair with buccal mucosal graft and gracilis muscle flaps as described recently by Palmer et al. may have the highest success rates (18).
Conclusions
Radiation-induced urethral stricture management is a challenge for the physician and patient. Urethroplasty procedures remain the mainstay of surgical treatment in these patients to avoid chronic urethral or suprapubic catheterization. The vast majority of strictures is located in the bulbomembranous urethra and is amenable to treatment with EPA. The use of graft tissue or flaps should be reserved for the most extensive strictures given the challenges of a fibrotic irradiated tissue environment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw 2014;12:686-718. [PubMed]
- Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007;177:2106-31. [PubMed]
- Jarosek SL, Virnig BA, Chu H, et al. Propensity-weighted Long-term Risk of Urinary Adverse Events After Prostate Cancer Surgery, Radiation, or Both. Eur Urol 2014. [Epub ahead of print]. [PubMed]
- Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:204-12. [PubMed]
- Hindson BR, Millar JL, Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: analysis of risk factors. Brachytherapy 2013;12:50-5. [PubMed]
- Glass AS, McAninch JW, Zaid UB, et al. Urethroplasty after radiation therapy for prostate cancer. Urology 2012;79:1402-5. [PubMed]
- Hofer MD, Zhao LC, Morey AF, et al. Outcomes after urethroplasty for radiotherapy induced bulbomembranous urethral stricture disease. J Urol 2014;191:1307-12. [PubMed]
- Bernstein EF, Sullivan FJ, Mitchell JB, et al. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg 1993;20:435-53. [PubMed]
- Turina M, Mulhall AM, Mahid SS, et al. Frequency and surgical management of chronic complications related to pelvic radiation. Arch Surg 2008;143:46-52; discussion 52. [PubMed]
- Gardner BG, Zietman AL, Shipley WU, et al. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol 2002;167:123-6. [PubMed]
- Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 2009;91:232-6. [PubMed]
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178:529-34; discussion 534. [PubMed]
- Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol 2006;176:2508-13. [PubMed]
- Mundy AR, Andrich DE. Urethral strictures. BJU Int 2011;107:6-26. [PubMed]
- Jackson MJ, Sciberras J, Mangera A, et al. Defining a patient-reported outcome measure for urethral stricture surgery. Eur Urol 2011;60:60-8. [PubMed]
- Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. J Urol 2000;163:1070-5. [PubMed]
- McAninch JW, Laing FC, Jeffrey RB Jr. Sonourethrography in the evaluation of urethral strictures: a preliminary report. J Urol 1988;139:294-7. [PubMed]
- Palmer DA, Buckley JC, Zinman LN, et al. Urethroplasty for High Risk, Long Segment Urethral Strictures with Ventral Buccal Mucosa Graft and Gracilis Muscle Flap. J Urol 2014. [Epub ahead of print]. [PubMed]
- Beard CJ, Propert KJ, Rieker PP, et al. Complications after treatment with external-beam irradiation in early-stage prostate cancer patients: a prospective multiinstitutional outcomes study. J Clin Oncol 1997;15:223-9. [PubMed]
- Meeks JJ, Brandes SB, Morey AF, et al. Urethroplasty for radiotherapy induced bulbomembranous strictures: a multi-institutional experience. J Urol 2011;185:1761-5. [PubMed]
- Herschorn S, Elliott S, Coburn M, et al. SIU/ICUD Consultation on Urethral Strictures: Posterior urethral stenosis after treatment of prostate cancer. Urology 2014;83:S59-70. [PubMed]
- Kane CJ, Tarman GJ, Summerton DJ, et al. Multi-institutional experience with buccal mucosa onlay urethroplasty for bulbar urethral reconstruction. J Urol 2002;167:1314-7. [PubMed]
- Markiewicz MR, DeSantis JL, Margarone JE 3rd, et al. Morbidity associated with oral mucosa harvest for urological reconstruction: an overview. J Oral Maxillofac Surg 2008;66:739-44. [PubMed]
- Stein R, Schröder A, Thüroff JW. Surgical atlas: Primary hypospadias repair with buccal mucosa. BJU Int 2006;97:871-89. [PubMed]
- Barbagli G, Montorsi F, Guazzoni G, et al. Ventral oral mucosal onlay graft urethroplasty in nontraumatic bulbar urethral strictures: surgical technique and multivariable analysis of results in 214 patients. Eur Urol 2013;64:440-7. [PubMed]


