Augmented urethroplasty with pseudospongioplasty in the treatment of penile strictures
Introduction
Many options exist for repairing anterior urethral strictures. Consequently, it is important for the treating surgeon to have a broad armamentarium encompassing various procedures as each patient presents with their own unique characteristics. Strictures involving the penile urethra generally require tissue transfer techniques using either grafts or flaps, alone or in combination. Grafts are preferred to flaps because of their lower morbidity, and decreased complexity, with similar efficacy (1-3). Flaps are associated with penile hematoma, skin necrosis, fistula formation, glans torsion and a higher incidence of sacculation. The remainder of this article will focus primarily on grafts.
In general, grafts can be placed ventrally, dorsally or laterally, in a 1- or 2-stage procedure. Ventral graft placement usually is avoided in the penile urethra because of the relative paucity of viable corpus spongiosal tissue necessary for vascularization and support. Instead, such grafts commonly are placed dorsally, using either an onlay or inlay technique. However, recent literature has reported successful penile urethral replacement using ventrally placed grafts supported by a pseudospongioplasty (4). The results appear to be comparable to ventral grafts placed in the bulbar urethra reinforced with a spongioplasty (5-7). Additionally these outcomes compare favorably to procedures using dorsally placed penile urethral grafts (8-11).
A review of the literature suggests that the stricture recurrence rate for urethral augmentation is approximately 10-20% at 5 years (1,2,5,12). However, the durability of these procedures may decrease over time, as shown by Andrich et al. who reported restricture rates as high as 58% at 15 years for augmented urethroplasties (12-14). Their cohort may reflect a different population, as patients in older series were more likely to undergo repetitive endoscopic procedures prior to reconstruction. In contrast, in contemporary series urethroplasty is performed sooner with a wider array of surgical options. It is therefore likely that current long-term outcomes will be improved. Indeed, Barbagli et al. reported an overall success rate of 73.8% in augmented anterior urethroplasties with a minimum of 6 years of follow-up at a median of 118 months (13).
Various grafts have been described to reconstruct the urethra. These include, oral mucosa (i.e., buccal and lingual tissue), genital skin (i.e., penile), extra genital skin (i.e., posterior auricular), bladder epithelium, and colonic mucosa (10,15-20). Buccal mucosa has prevailed and is currently regarded as the preferred graft in urethral reconstruction. It is unlikely that this will change until improvements in regenerative engineering provide us with adequate and reliable autologous tissue.
Buccal grafts
The use of buccal mucosa was originally described by Humby in 1941 as a single stage procedure in an 8-year-old hypospadiac cripple (21). However, it wasn’t until 1992, after Dr. Bürger’s preliminary report on six patients, that enthusiasm for buccal mucosa as an alternative for tissue transfer in urethral reconstruction was born (22). Buccal mucosa has several distinct characteristics that make it advantageous. These include its ease of harvest, its thick resilient epithelium, which facilitates handling, and its thin but densely vascular lamina propria, which provides an excellent milieu for effective imbibition and inosculation. In addition, unlike skin, buccal mucosa grafts naturally exist in a moist environment, making them more adaptable to urethral transfer. They also appear to be resistant to inflammatory skin diseases such as lichen sclerosus et atrophicus and vitiligo. Grafts can be placed ventrally, dorsally or laterally, to augment the urethra. The location of placement is at the discretion of the reconstructive surgeon—a decision based on the intraoperative stricture characteristics and/or surgeon comfort with each technique.
Graft placement
Dorsal
Dorsal grafts may be used both in the bulbar and penile urethral segments. Dorsal grafts are mechanically supported and vascularized by the underlying corpus cavernosa to which they are secured (9,23). They theoretically have a lower risk of sacculation and subsequent urethral diverticulum (23). A disadvantage of dorsally placed grafts is that they require more extensive circumferential dissection to mobilize the urethra. Additionally, as the penile urethral dissection advances distally, the corpus spongiosum becomes more adherent to the tunica albuginea with enhanced emissary vasculature, making dorsal spongiosal dissection more difficult. In such cases, a dorsal inlay is preferable to an onlay technique (24).
Lateral
Similarly, laterally placed grafts have been described in both the bulbar and penile urethra (5,25). In laterally placed grafts, dissection is carried out only on one side of the urethra leaving the contralateral blood supply intact. Laterally placed grafts have been reported to have similar success rates as dorsal or ventrally placed grafts in the bulbar urethra (5).
Ventral
In 1996, Drs. Morey and McAninch described the use of ventrally placed buccal mucosa grafts in the bulbar urethra with a spongioplasty for graft support and take (6). This technique has become one of the most commonly performed reconstructions for bulbar strictures not amenable to an anastomotic repair. The ventral approach allows for direct exposure of the stricture and ease of suturing. However, the use of ventral grafts in the penile urethra traditionally has been limited due to the paucity of corpora spongiosum—a consequence of its concentric configuration, as compared to its ventrally eccentric arrangement in the bulb. Previously, ventrally placed grafts in the penile urethra were thought to have a reduced survival because of an inadequately vascularized host bed (1,6,26-28). However, the incorporation of a pseudospongioplasty provides a well-vascularized milieu for successful graft take in this location.
Pseudospongioplasty
In areas where the corpus spongiosum is inadequate for buccal mucosa graft coverage, such as in the penile urethra, a pseudospongioplasty can be used for the reconstruction. This technique incorporates periurethral flaps of tunica dartos and Buck’s fascia for graft coverage, allowing for successful ventral graft placement.
Anatomy of periurethral tissue
The penile urethra is covered by Buck’s fascia and tunica dartos. Buck’s fascia was originally described in 1848 as a distinct membranous sheath investing the penis, that was appreciated in a patient who presented with a periurethral abscess (29). Buck’s fascia is the deep penile fascia that envelops the tunica albuginea of the corporal bodies, and ventrally splits to envelop the corpus spongiosum within a separate compartment. Buck’s fascia carries the deep dorsal artery and vein. It provides support for the more superficial and loose tunica dartos that is responsible for the high degree of mobility of the penile skin. The tunica dartos carries a longitudinal-coursing anastomotic network of vessels.
The robust and resilient blood supply of the tunica dartos and Buck’s fascia arise from the superficial and deep external pudendal artery (30). The combination of the tunica dartos and Buck’s fascia provides sufficient vascularity and reinforcement for graft survival and support.
Technique
The penile urethra is exposed using either a ventral subcoronal or longitudinal incision that is carried down to the corpus spongiosum. Periurethral flaps are fashioned by either longitudinally incising or proximally mobilizing the tunica dartos and Buck’s fascia. Passing an 18 French red rubber catheter transurethrally identifies the distal aspect of the stricture. A ventral stricturotomy is then performed (Figure 1), and extended proximally and distally to healthy tissue. Bougie-à-boule dilators are used to calibrate the urethra, ensuring a sufficiently patent lumen. Flexible cystoscopy is carried out to evaluate the entire urethra and bladder. An appropriately sized buccal mucosa graft is then harvested, defatted, and sutured to the lateral edges of the incised urethra using running 5-0 polydioxanone sutures (Figure 2). Interrupted sutures are placed at the proximal and distal anastomoses to limit undue apical narrowing. Once the graft is properly secured, the final Foley catheter is placed (Figure 3). The previously created periurethral flaps are then either sutured together in the midline or advanced distally, in either case avoiding overlying suture lines. This ensures complete coverage of the buccal graft with well-vascularized tissue comprised of both tunica dartos and Buck’s fascia (Figure 4). The graft can be secured distally to the superficial subcoronal tissue to limit its mobility. The penile skin is then re-approximated, again taking care not to overlap suture lines. A compression dressing (i.e., Coban) is used for one week to promote fixation of the graft to supporting tissues, allowing for proper imbibition and inosculation. Penile elevation is helpful in minimizing postoperative edema. A Foley catheter is left in place for 3 weeks and a voiding cystourethrogram is performed on the day of catheter removal.
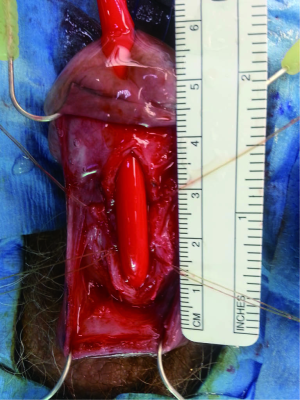
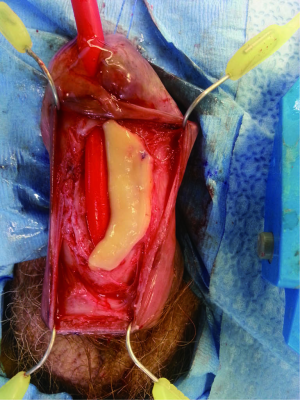
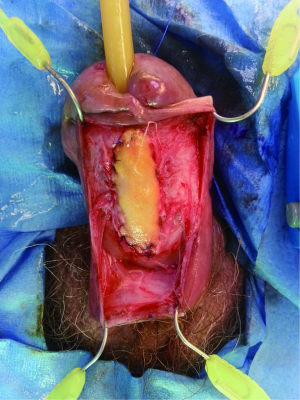
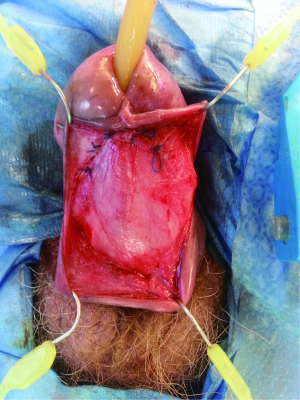
Graft coverage and perfusion
There are several observations that lend credence to the ability of the periurethral tissue to provide a suitable blood supply necessary for survival of buccal mucosa grafts placed ventrally. First, Buck’s fascia is the primary fascia providing support to the vascular tunica dartos pedicle in circular fasciocutaneous penile flaps. The robust arterial supply and versatility of the periurethral tissue is confirmed by the generous mobilization and sufficient length achieved by fasciocutaneous flaps to repair complex anterior urethral strictures (26). Second, the tunica dartos provides an excellent host bed for both full-thickness and split-thickness skin grafts (31,32). This is evident, even when the urethra is deficient as with hypospadias, by the successful use of tunica dartos flaps for coverage of the ventral suture line (33). Given evidence of the robust and predictable blood supply to the periurethral tissue it would be reasonable to conclude that a ventral onlay buccal mucosa graft with pseudospongioplasty would be as successful as conventional spongioplasty.
Pseudospongioplasty outcomes
Recently Drs. Armenakas and Morey reported their combined results using pseudospongioplasty for distal bulbar and penile ventral buccal mucosa graft urethroplasties (4). A total of 120 patients were evaluated at a mean follow-up of 40 months. The success rates of conventional spongioplasty and pseudospongioplasty were equivalent (84% and 80% respectively, P=0.645). Failure was defined as the need for a subsequent procedure and occurred overall in 18% with a mean time to failure of 13 months in both groups. Furthermore, success rates were similar despite longer strictures in the pseudospongioplasty group. The mean stricture length was 4.3 cm for the conventional spongioplasty compared to 5.8 cm for the pseudospongioplasty (P=0.028). Stricture length was not significantly different between the successful and failed urethroplasties (mean =5.2 cm). Others have also been successful with similar techniques. In a smaller series, with shorter follow-up, Jinga et al. (34) reported on 27 penile strictures treated with ventral buccal mucosa grafts covered with lateral dartos flaps for a success rate of 88.9% (24/27) at a mean of 21 months (range, 4-35 months). These data support the similar efficacy seen for pseudospongioplasty compared to conventional spongioplasty.
Pseudospongioplasty outcomes are also comparable to other graft augmentation urethroplasties performed in the penile urethra. In a recent systematic review of graft augmentation penile urethroplasty, 21 studies reporting outcomes of 1-stage urethroplasty were reviewed. A total of 432 patients with an average of 32.8 months follow-up had a mean success rate of 75.68% (2). There was wide variance in the success rate between studies. When analyzing only series with more than 40 patients, success rates ranged from 67-100% (2,35-37).
Conclusions
Standard spongioplasty should be performed whenever possible. However, in instances where the corpus spongiosum is inadequate for graft coverage, ventral buccal mucosa graft placement with pseudospongioplasty is a reliable option. This technique is best suited for penile urethral strictures. Its advantages include the need for minimal dissection, its ease of transfer, and its predictable robust blood supply. Initial results using the pseudospongioplasty for graft coverage are comparable to standard spongioplasty results within the bulbar urethra, as well as results using alternative graft techniques in the penile urethra.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wessells H, McAninch JW. Current controversies in anterior urethral stricture repair: free-graft versus pedicled skin-flap reconstruction. World J Urol 1998;16:175-80. [PubMed]
- Mangera A, Patterson JM, Chapple CR. A systematic review of graft augmentation urethroplasty techniques for the treatment of anterior urethral strictures. Eur Urol 2011;59:797-814. [PubMed]
- Dubey D, Vijjan V, Kapoor R, et al. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol 2007;178:2466-9. [PubMed]
- Cordon BH, Zhao LC, Scott JF, et al. Pseudospongioplasty using periurethral vascularized tissue to support ventral buccal mucosa grafts in the distal urethra. J Urol 2014;192:804-7. [PubMed]
- Barbagli G, Palminteri E, Guazzoni G, et al. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol 2005;174:955-7; discussion 957-8. [PubMed]
- Morey AF, McAninch JW. When and how to use buccal mucosal grafts in adult bulbar urethroplasty. Urology 1996;48:194-8. [PubMed]
- Heinke T, Gerharz EW, Bonfig R, et al. Ventral onlay urethroplasty using buccal mucosa for complex stricture repair. Urology 2003;61:1004-7. [PubMed]
- Iselin CE, Webster GD. Dorsal onlay urethroplasty for urethral stricture repair. World J Urol 1998;16:181-5. [PubMed]
- Barbagli G, Selli C, Tosto A, et al. Dorsal free graft urethroplasty. J Urol 1996;155:123-6. [PubMed]
- Markiewicz MR, Lukose MA, Margarone JE 3rd, et al. The oral mucosa graft: a systematic review. J Urol 2007;178:387-94. [PubMed]
- Aldaqadossi H, El Gamal S, El-Nadey M, et al. Dorsal onlay (Barbagli technique) versus dorsal inlay (Asopa technique) buccal mucosal graft urethroplasty for anterior urethral stricture: a prospective randomized study. Int J Urol 2014;21:185-8. [PubMed]
- Andrich DE, Dunglison N, Greenwell TJ, et al. The long-term results of urethroplasty. J Urol 2003;170:90-2. [PubMed]
- Barbagli G, Kulkarni SB, Fossati N, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol 2014;192:808-13. [PubMed]
- Breyer BN, McAninch JW, Whitson JM, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol 2010;183:613-7. [PubMed]
- Barbagli G, Palminteri E, Rizzo M. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa in adult bulbourethral strictures. J Urol 1998;160:1307-9. [PubMed]
- Schwentner C, Seibold J, Colleselli D, et al. Dorsal onlay skin graft urethroplasty in patients older than 65 years. Urology 2010;76:465-70. [PubMed]
- Manoj B, Sanjeev N, Pandurang PN, et al. Postauricular skin as an alternative to oral mucosa for anterior onlay graft urethroplasty: a preliminary experience in patients with oral mucosa changes. Urology 2009;74:345-8. [PubMed]
- Hendren WH, Reda EF. Bladder mucosa graft for construction of male urethra. J Pediatr Surg 1986;21:189-92. [PubMed]
- Marzorati G, Ghinolfi G, Pachera F, et al. Bladder and buccal mucosa graft in urethral stricture reconstruction. Urologia 2008;75:177-9. [PubMed]
- Xu YM, Qiao Y, Sa YL, et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol 2009;182:1040-3. [PubMed]
- Humby G, Twistington Higgins T. A one-stage operation for hypospadias. Br J Surg 1941;29:84-92.
- Bürger RA, Müller SC, el-Damanhoury H, et al. The buccal mucosal graft for urethral reconstruction: a preliminary report. J Urol 1992;147:662-4. [PubMed]
- Barbagli G, Selli C, di Cello V, et al. A one-stage dorsal free-graft urethroplasty for bulbar urethral strictures. Br J Urol 1996;78:929-32. [PubMed]
- Asopa HS, Garg M, Singhal GG, et al. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology 2001;58:657-9. [PubMed]
- Kulkarni S, Barbagli G, Sansalone S, et al. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int 2009;104:1150-5. [PubMed]
- McAninch JW, Morey AF. Penile circular fasciocutaneous skin flap in 1-stage reconstruction of complex anterior urethral strictures. J Urol 1998;159:1209-13. [PubMed]
- Morey AF, Duckett CP, McAninch JW. Failed anterior urethroplasty: guidelines for reconstruction. J Urol 1997;158:1383-7. [PubMed]
- Patterson JM, Chapple CR. Surgical techniques in substitution urethroplasty using buccal mucosa for the treatment of anterior urethral strictures. Eur Urol 2008;53:1162-71. [PubMed]
- Buck G. A New Feature in the Anatomical Structure of the Genito-Urinary Organs not hitherto described. Transactions of the American Medical Association 1848;1:367-71.
- Buckley J, McAninch J. Distal penile circular fasciocutaneous flap for complex anterior urethral strictures. BJU Int 2007;100:221-31. [PubMed]
- Meeks JJ, Erickson BA, Gonzalez CM. Staged reconstruction of long segment urethral strictures in men with previous pediatric hypospadias repair. J Urol 2009;181:685-9. [PubMed]
- Thakar HJ, Dugi DD 3rd. Skin grafting of the penis. Urol Clin North Am 2013;40:439-48. [PubMed]
- Snodgrass W, Yucel S. Tubularized incised plate for mid shaft and proximal hypospadias repair. J Urol 2007;177:698-702. [PubMed]
- Jinga V, Hurduc M, Voinescu V, et al. Ventral buccal mucosa graft urethroplasty for penile urethral strictures: a predictable failure? Chirurgia (Bucur) 2013;108:245-9. [PubMed]
- Mathur RK, Himanshu A, Sudarshan O. Technique of anterior urethra urethroplasty using tunica albuginea of corpora cavernosa. Int J Urol 2007;14:209-13. [PubMed]
- Barbagli G, Morgia G, Lazzeri M. Retrospective outcome analysis of one-stage penile urethroplasty using a flap or graft in a homogeneous series of patients. BJU Int 2008;102:853-60. [PubMed]
- Andrich DE, Mundy AR. Substitution urethroplasty with buccal mucosal-free grafts. J Urol 2001;165:1131-3; discussion 1133-4. [PubMed]


