Use of overlapping buccal mucosa graft urethroplasty for complex anterior urethral strictures
Introduction
Urethroplasty is the gold standard treatment for anterior urethral strictures. With success rates eclipsing 90% in many series, most patients can be cured of their stricture-related voiding problems with a single, straightforward, well tolerated operation. However, a select subset of urethral strictures are more complex, requiring more involved repairs with lower success rates and the potential for increased complications and decreased quality of life (1,2).
Urethroplasty success is predicated on adoption of one or both of the following principles: excision and augmentation. For excisional urethroplasty, the diseased segment is excised, and the healthy ends anastomosed. For augmentation urethroplasty, the diseased segment is incised and the lumen widened by augmenting the incised urethra with a graft or flap of substitute epithelium (typically oral mucosa or penile skin, respectively). While most strictures are amenable to single stage repair by following these principles, some strictures are too long for simple excision and/or have a urethral plate which is unfit for augmentation, thus greatly increasing the complexity of repair required (Figure 1).
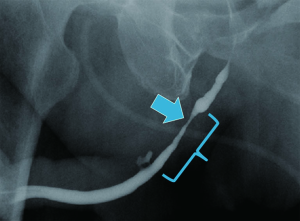
The conventional reconstruction technique for anterior urethral strictures unfit for single stage excision and/or augmentation is staged urethroplasty (3-5). During stage 1, the diseased urethra is incised or excised and a proximal urethrostomy is matured. A neo-urethral plate is then created by fixing a graft of buccal mucosa (BMG) or hairless skin to the neomeatus and adjacent incised skin edges. Several months later, the neourethra is separated from the skin and tubularized. While this well-established approach is certainly acceptable for the management of complex anterior strictures, it does have numerous unique drawbacks. Multi-stage urethroplasty is more burdensome for the patient due to the bed-rest and bolstered dressings required after the first stage, the long interval of graft maturation between stages (with attendant cosmetic, sexual, and voiding implications of a proximal urethrostomy and/or suprapubic tube), and the potential for a second-stage operation with the requisite cost, convalescence, and catheter drainage for several more weeks. In some series, 50% or more of the men who underwent first stage urethroplasty did not return for second-stage urethroplasty (3,6,7). Additionally, 2-staged urethroplasty often becomes multi-staged, given the 18-45% rate of additional revision procedures needed for neourethral stenosis after the second stage (3,6,7). Given these drawbacks, single stage reconstruction with overlapping buccal mucosal graft urethroplasty (OBMGU) offers a potential alternative to multi-stage urethroplasty for complex anterior urethral strictures.
Rationale for OBMGU
Adequate graft support
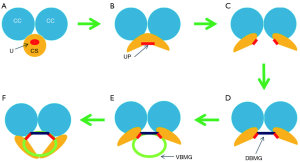
Success with tissue grafting requires a healthy recipient bed where the graft matures during the 2-step process of imbibition and inosculation. Graft-augmented urethroplasty also requires a urethral plate of adequate width to allow anastomosis of the graft to either end of the plate. If the urethral plate is too narrow, a wide graft must be used to achieve a normal neourethral caliber, placed in a configuration which approximates that of a complete tube (Figure 2). Such an approach is problematic, given the historically poor results obtained with tubularized repairs (8) and the challenge of performing a spongioplasty to support wide, ventrally placed grafts (9). Using dual, overlapping grafts these problems can be avoided. The dorsal graft (supported by the ventral corporal bodies) reconstitutes the urethral plate and the ventral graft spans the entire length of the stricture. The overlapping concept allows for a narrower ventral graft, thus avoiding tubularized grafts and facilitating conventional spongioplasty for bulbar strictures (Figure 3) (10) or “pseudospongioplasty” in the pendulous urethra where the corpus spongiosum is less robust (9).

Avoidance of urethral transection
Despite the excellent long term results of excision and primary anastomosis (EPA) for short bulbar strictures (11), some authors remain concerned about the potential for troublesome sexual side effects caused by disruption of the neurovascular structures of the corpus spongiosum when the full-thickness spongiosal complex is transected and excised (12,13). OBMGU has been shown to be an effective alternative to EPA for short bulbar strictures when concerns about urethral transection exist (13-16). While we believe that the potential for neurovascular related sexual dysfunction following EPA is minimal in uncomplicated patients, men with known vascular compromise of the corpus spongiosum (i.e., those with multiple urethral strictures, prior urethral transection, indwelling artificial urinary sphincter, history of hypospadias, and/or prior pelvic radiation therapy) can benefit from the non-transecting technique employed during OBMGU (discussed below).
Technique for OBMGU
Multiple technical nuances for OBMGU have been reported (10,16,17). We prefer a modification of the technique originally reported by Palminteri et al. (16) which is a hybrid of two well established techniques: the ventral onlay procedure popularized by Elliott et al. (18) and the ventral sagittal approach for dorsal graft placement originally described by Asopa et al. (19,20). For all patients, stricture anatomy is delineated with retrograde/voiding cystourethrography and flexible cystoscopy. Urethral catheters, when present, are removed at least 6 weeks preoperatively to allow for maturation of the stricture, thus enabling intraoperative identification of the diseased segment (21). Preoperative suprapubic cystostomy tubes are placed selectively for those men with debilitating lower urinary tract symptoms (LUTS) and/or urinary retention.
Patient positioning is determined by the location of the stricture (supine for pendulous strictures, dorsal lithotomy for bulbar strictures). BMG harvest and preparation is performed utilizing conventional techniques (22,23) using a two-team approach when feasible. The urethra is exposed via a ventral midline incision. Cystoscopic placement of a guide wire across the stricture facilitates safe and precise ventral stricturotomy beginning at the distal end of the stricture and extended proximally until normal caliber urethra is reached. The tissue quality of the urethral plate is examined and the stricture length and width assessed.
For obliterative urethral plate segments (<5 mm wide) and those with severe spongiofibrosis, the urethral plate and underlying fibrotic corpus spongiosum are focally excised. The urethral plate is then reconstituted with a fenestrated dorsal BMG, spread-fixed to the corporal bodies with interrupted 5-0 polyglactin suture. Urethral plate segments 5-10 mm wide with healthy corpus spongiosum are incised in the dorsal midline to the level of the tunica albuginea and lateralized approximately 1.5 cm. The resultant 1.5 cm wide elliptical defect in the urethral plate is then reconstituted with a fenestrated dorsal BMG fixed to the medial edges of the urethral plate and quilted to the ventral corporal tunica with interrupted 5-0 polyglactin suture. The widened urethral plate is then supplemented with an appropriately tailored ventral BMG secured across the entire length of the stricturotomy with running 5-0 polydioxanone suture over a 16 Fr urethral catheter. The combined width of the dorsal BMG, residual urethral plate and ventral BMG should equal 30 mm, resulting in a final neo-urethral caliber of approximately 30 Fr. Local tissues are used to support the ventral BMG: corpus spongiosum, tunica dartos, or tunica vaginalis, depending on location and tissue availability. Wounds are closed in three layers and drains are not routinely necessary. Most patients are discharged after 23 hours of observation and return for catheter removal in 2-3 weeks after a voiding cystourethrogram confirms a well-healed repair.
Outcomes of OBMGU
The first reported experience with OBMGU was published by Palminteri et al. in 2008 (16). The authors described a non-transecting, non-excisional reconstruction technique for bulbar strictures too long for EPA and too narrow for conventional onlay grafting. They hypothesized that urethral transection may compromise the spongiosal vascular supply, thus risking postoperative sexual dysfunction and that urethral excision violates the axial integrity of the urethra-spongiosal complex, thus risking postoperative chordee. In their initial report, 45 of the 48 bulbar-only OBMGU were successful at a mean 22 months after reconstruction. Average stricture length was 3.65 cm (range, 2-10 cm) and average graft dimensions were approximately 6 cm × 2 cm. No postoperative complications were reported and all five recurrences presented within the first postoperative year (16).
Continued work by Palminteri et al. demonstrated durable results of OBMGU with an 88% success rate in an expanded series of 73 bulbar strictures with a minimum follow up of 1 year (mean 49 months) (15). Sexual complications were avoided in this series and 6 of the 9 recurrent strictures were salvaged with internal urethrotomy. Late recurrences were seen, however, with three patients demonstrating stricture recurrence between 2 and 3 years postoperatively. The authors concluded that OMBGU is a valuable technique for augmentation urethroplasty in patients with severe strictures in whom preservation of the urethral plate is desired (15).
While the work by Palminteri et al. (15), was limited to bulbar strictures, a subsequent multi-institutional study applied OBMGU to all anterior strictures regardless of location (bulbar and/or pendulous) (10). In their series of 36 patients who underwent OBMGU, 39% of strictures involved the pendulous urethra. Overall mean stricture length was 4.5 cm and 25% of the men had a history of either hypospadias or lichen sclerosis. Despite a more complex group of strictures compared to those treated by Palminteri et al. (15), a similar success rate of 89% was observed a mean 16 months after urethroplasty, suggesting that OBMGU is a reliable procedure for both bulbar and pendulous urethral strictures (10).
In a more recent report, Gelman et al. described their initial experience with an alternative technique for OBMGU which involved circumferential urethral dissection and dorsal urethrotomy (17). Once exposed via the dorsal urethrotomy, obliterative urethral mucosa was excised until non-fibrotic spongy tissue was seen, thus removing the scarred, unusable urethral plate while sparing the underlying vascular spongiosum and avoiding full-thickness spongiosal transection. The excised urethral plate was reconstituted with a ventral BMG quilted to the spongiosum. A second BMG was then quilted to the ventral corporal bodies and the repair completed over a 16 Fr urethral catheter similar to a conventional dorsal onlay BMG urethroplasty. This novel technique showed promising results with 17 of the 18 patients having cystoscopic urethral patency 4 months after surgery and freedom for further procedures a mean 50 months postoperatively. The authors preferred the dorsal approach because only one urethrotomy was required, thus limiting dissection through the spongiosum and perhaps decreasing the risk of vascular compromise across the incised portion of the urethra (17).
Alternatives to OBMGU
As the above studies have demonstrated, OBMGU is an innovative technique which can be applied across the broad spectrum of complex anterior urethral stricture disease. However, some strictures may present unique challenges for which alternative techniques may offer advantages over OBMGU. For example, when the corpus spongiosum is severely scarred or absent (as seen after destructive penetrating trauma or multiple failed attempts at urethroplasty), there may be insufficient local tissue to support the ventrally placed BMG. In such scenarios, the combination of a dorsally placed BMG with a ventrally placed penile skin flap has been shown by multiple authors to be a reliable alternative to multi-stage reconstruction (24-26). Disadvantages of this “flap on graft” approach include the additional dissection required for penile skin flap mobilization and the need for penile skin of adequate quality and amount. Men with a history of lichen sclerosis and/or hypospadias often have deficient or diseased foreskin precluding flap construction, a relatively common situation noted in a previously published OBMGU series (10).
A simple alternative to OBMGU for severely narrowed (but not completely obliterated) bulbar strictures was recently published by Barbagli et al. (27). In this report, tubularized grafts were avoided by deliberately leaving a gap between one side of the urethral plate and the ipsilateral margin of the BMG, thus suturing the graft directly to the corpus spongiosum and relying on epithelial ingrowth to reconstitute the narrowed urethral plate. While the authors reported similarly favorable outcomes compared to those with wider urethral plates who underwent conventional ventral BMG urethroplasty (27), we have found that the addition of the second, dorsally placed BMG permits use of narrower grafts, preserves the ability to complete mucosa-to-mucosa, graft-to-plate anastomoses, and results in potentially lower rates of urinary extravasation, sacculation, and recurrent stricture formation (10).
Conclusions
Effective management of patients with complex anterior urethral strictures requires a diverse armamentarium of reconstructive techniques including various excisional, incisional, and augmentation based procedures. Recent studies indicate that OBMGU is a reliable technique for the single stage reconstruction of anterior urethral strictures too long for excisional urethroplasty, too complex for simple only augmentation, and for patients in whom the onerous process of multi-stage reconstruction is unacceptable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Disclaimer: The view(s) expressed herein are those of the author and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army and Department of Defense or the U.S. Government.
References
- Myers JB, McAninch JW, Erickson BA, et al. Treatment of adults with complications from previous hypospadias surgery. J Urol 2012;188:459-63. [PubMed]
- Levine LA, Strom KH, Lux MM. Buccal mucosa graft urethroplasty for anterior urethral stricture repair: evaluation of the impact of stricture location and lichen sclerosus on surgical outcome. J Urol 2007;178:2011-5. [PubMed]
- Elliott SP, Eisenberg ML, McAninch JW. First-stage urethroplasty: utility in the modern era. Urology 2008;71:889-92. [PubMed]
- Kozinn SI, Harty NJ, Zinman L, et al. Management of complex anterior urethral strictures with multistage buccal mucosa graft reconstruction. Urology 2013;82:718-22. [PubMed]
- Williams JL, Crawford BS. A method of urethroplasty for urethral strictures. Br J Urol 1968;40:712-6. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. Clinical outcome and quality of life assessment in patients treated with perineal urethrostomy for anterior urethral stricture disease. J Urol 2009;182:548-57. [PubMed]
- Peterson AC, Palminteri E, Lazzeri M, et al. Heroic measures may not always be justified in extensive urethral stricture due to lichen sclerosus (balanitis xerotica obliterans). Urology 2004;64:565-8. [PubMed]
- Wiener JS, Sutherland RW, Roth DR, et al. Comparison of onlay and tubularized island flaps of inner preputial skin for the repair of proximal hypospadias. J Urol 1997;158:1172-4. [PubMed]
- Cordon BH, Zhao LC, Scott JF, et al. Pseudospongioplasty using periurethral vascularized tissue to support ventral buccal mucosa grafts in the distal urethra. J Urol 2014;192:804-7. [PubMed]
- Hudak SJ, Lubahn JD, Kulkarni S, et al. Single-stage reconstruction of complex anterior urethral strictures using overlapping dorsal and ventral buccal mucosal grafts. BJU Int 2012;110:592-6. [PubMed]
- Eltahawy EA, Virasoro R, Schlossberg SM, et al. Long-term followup for excision and primary anastomosis for anterior urethral strictures. J Urol 2007;177:1803-6. [PubMed]
- Andrich DE, Mundy AR. Non-transecting anastomotic bulbar urethroplasty: a preliminary report. BJU Int 2012;109:1090-4. [PubMed]
- Palminteri E, Berdondini E, De Nunzio C, et al. The impact of ventral oral graft bulbar urethroplasty on sexual life. Urology 2013;81:891-8. [PubMed]
- Palminteri E, Berdondini E, Fusco F, et al. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 2012;79:695-701. [PubMed]
- Palminteri E, Berdondini E, Shokeir AA, et al. Two-sided bulbar urethroplasty using dorsal plus ventral oral graft: urinary and sexual outcomes of a new technique. J Urol 2011;185:1766-71. [PubMed]
- Palminteri E, Manzoni G, Berdondini E, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol 2008;53:81-9. [PubMed]
- Gelman J, Siegel JA. Ventral and dorsal buccal grafting for 1-stage repair of complex anterior urethral strictures. Urology 2014;83:1418-22. [PubMed]
- Elliott SP, Metro MJ, McAninch JW. Long-term followup of the ventrally placed buccal mucosa onlay graft in bulbar urethral reconstruction. J Urol 2003;169:1754-7. [PubMed]
- Asopa HS, Garg M, Singhal GG, et al. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology 2001;58:657-9. [PubMed]
- Pisapati VL, Paturi S, Bethu S, et al. Dorsal buccal mucosal graft urethroplasty for anterior urethral stricture by Asopa technique. Eur Urol 2009;56:201-5. [PubMed]
- Terlecki RP, Steele MC, Valadez C, et al. Urethral rest: role and rationale in preparation for anterior urethroplasty. Urology 2011;77:1477-81. [PubMed]
- Dubey D, Kumar A, Mandhani A, et al. Buccal mucosal urethroplasty: a versatile technique for all urethral segments. BJU Int 2005;95:625-9. [PubMed]
- Duckett JW, Coplen D, Ewalt D, et al. Buccal mucosal urethral replacement. J Urol 1995;153:1660-3. [PubMed]
- Erickson BA, Breyer BN, McAninch JW. Single-stage segmental urethral replacement using combined ventral onlay fasciocutaneous flap with dorsal onlay buccal grafting for long segment strictures. BJU Int 2012;109:1392-6. [PubMed]
- Gelman J, Sohn W. 1-stage repair of obliterative distal urethral strictures with buccal graft urethral plate reconstruction and simultaneous onlay penile skin flap. J Urol 2011;186:935-8. [PubMed]
- Morey AF. Urethral plate salvage with dorsal graft promotes successful penile flap onlay reconstruction of severe pendulous strictures. J Urol 2001;166:1376-8. [PubMed]
- Barbagli G, Montorsi F, Guazzoni G, et al. Ventral oral mucosal onlay graft urethroplasty in nontraumatic bulbar urethral strictures: surgical technique and multivariable analysis of results in 214 patients. Eur Urol 2013;64:440-7. [PubMed]


