7-flap perineal urethrostomy
Introduction
Perineal urethrostomy (PU) has existed in its current form since the late 1960s. This procedure diverts the bulbar urethra distal to the external urinary sphincter into the perineum and most often bypasses the pendulous urethra (1). Due to the ever-increasing sophistication of endoscopic and operative urethral reconstructive techniques over the past five decades, the role of PU has most commonly been limited to the first-stage of a two-stage urethroplasty or employed for highly refractory cases of urethral stricture disease (2,3). In this review, we highlight the indications for PU performance, and further summarize both conventional and contemporary procedural outcomes that have been described with successful PU creation. We also review the technique and outcomes of “7-flap” PU, a novel technique developed by the authors that can be utilized in highly refractory cases of urethral stricture disease.
Indications for PU
Performed in the setting of anterior urethral stricture disease, the indications for PU commonly include complicated lichen sclerosis (LS) (3), the first-stage of a complex hypospadias repair, or failure of a prior urethroplasty (4). PU has also been utilized for patients with pan-urethral stricture disease or those sustaining traumatic urethral injuries. Recently, the role of PU has expanded to relative indications such as for men with advanced age and/or those who do not desire extensive urethral reconstruction (5). However, patients with proximal urethral strictures or bladder neck contractures should not be candidates for PU creation. Additionally, placement of an artificial urinary sphincter is technically challenging following PU creation, so men with poor sphincteric function due to prior prostate surgery should be approached cautiously. Proper consideration must be given to any surgical candidate’s quality of life, expectations, and voiding mechanics when considering PU as a treatment option. Lastly, effects on patient’s ejaculatory function and perineal appearance should also be fully disclosed during pre-operative counseling.
Conventional PU: technique and outcomes
The conventional technique for PU was meticulously described by Blandy in 1968, and at the time, his description was admittedly an adaptation of an established procedure popularized by Leadbetter (1). However, a distinguishing feature of Blandy’s procedure was the utilization of an elastic, mobile scrotal skin flap in lieu of perineal skin in order to complete the urethrocutaneous anastomosis. Despite being almost fifty years old, many urologists still perform PU as described by Blandy in contemporary urologic practice.
Blandy’s description of conventional urethroplasty involved an inverted Y-shaped perineal incision with mobilization of the scrotal flap towards the bulbar urethral segment. The urethra was first opened, followed by an incision of the strictured urethra and identification of the verumontanum. The scrotal flap and remaining skin were finally sewn to the edge of the flayed bulbar urethra with a non-absorbable suture, thus completing the urethrostomy. This fairly straightforward procedure has been the mainstay of PU ever since (4).
In 1971, Blandy published an updated, retrospective PU experience using his previously described technique (6). None of the 70 reported cases, including 51 (73%, 51/70) who went on for further completion of a second stage Johansen urethroplasty had reported recurrent strictures, incontinence or impotence during a median 3 year follow up. Bleeding from the spongiosum, sloughing of the scrotal flap tip, bridging of the opposed suture lines, and fistula formation were the major complications encountered.
In a large, retrospective contemporary series with a median follow up of 22 months, McAninch and colleagues reported favorable outcomes in 38 men undergoing conventional PU due to profound urethral stricture disease (7). Although the location and etiology of stricture varied, stricture following hypospadias repairs and LS were the most common etiologies for men undergoing PU. Reflecting the complexity of urethral stricture disease, more than half of the series (61%, 23/38) had undergone prior urethroplasty. Citing overall happiness with voiding patterns following first-stage urethroplasty with PU creation, only nine patients (24%, 9/38) elected to proceed with second-stage urethroplasty. Interestingly, as the total number of urethroplasties increased over the study period, so too did the number of definitive first-stage procedures.
The largest and perhaps most comprehensive retrospective analysis of conventional PU was performed recently by Barbagli and associates on 173 patients over a 29-year period (8). With a median follow up of 62 months, the investigators reported their overall success rate, defined as freedom from post-operative instrumentation, at 70%. Age, stricture length greater than 6 cm, and stricture after hypospadias repair were all favorable prognostic indicators for successful PU, while those patients with a history of infectious or traumatic stricture etiology were at the highest risk of PU failure. Mirroring the results of McAninch’s series, nearly 75% of the patients deferred the option for second stage urethroplasty and elected to remain with PU. A similar series published in the same year examined a large, multi-institutional European experience with 215 LS patients having complex urethral stricture disease. A notable proportion of patients (21.8%, 47/215) underwent definitive PU using a conventional approach with a similar 72% success rate (9).
The observation that patients who undergo PU as part of a planned two-stage urethroplasty may safely forego the second stage, has raised the question of whether “heroic” attempts to establish urethral continuity are warranted. The 11-year Duke University experience revealed favorable results with PU in 44 of 63 patients who underwent only the first-stage of a planned two-stage urethroplasty. At 38.5 months follow-up, no patient needed any re-operative procedure or dilation and all patients reported high levels of happiness following surgery (10).
Despite the relative paucity of data, existing retrospective series that evaluate conventional PU generally demonstrate excellent results in terms of stricture recurrence and quality of life independent of stricture etiology. In patients undergoing conventional PU, stenosis of the PU is a rare occurrence that is best avoided by the surgeon’s determination of adequate scrotal flap length prior to making the Y-shaped perineal incision. However, as PU becomes an increasingly acceptable treatment in the reconstructive armamentarium for more complex cases, patient factors such as obesity or pan-urethral stricture disease can make conventional PU performance challenging. Recognizing this limitation, the study authors report a detailed description and review of outcomes of a novel 7-flap PU technique that does not require an estimation of flap length prior to incision (11).
7-flap PU: technique
Performance of PU (Figure 1) can be technically challenging in patients with either extensive pan-urethral stricture disease or a long skin-to urethra distance (e.g., obese patients). In an effort to treat such complicated cases effectively, the study authors recently published an initial experience using 7-flap PU, a technique that allows the surgeon to expose the proximal urethra prior to creating a custom-tailored skin flap for urethral attachment.
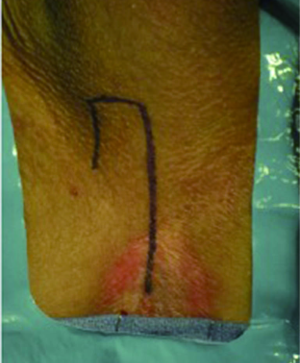
7-flap PU is performed with the patient in the dorsal lithotomy position. A squared 7-shaped figure is marked on the perineum with the vertical base of the “7” on the midline (Figure 2), thus replacing the inverted “Y” incision of the conventional approach. Initially, only the midline portion of the “7” is developed via a vertical perineal incision. The subcutaneous tissues are then divided and the bulbospongiosus muscle is divided in the midline. A self-retaining retractor (e.g., LoneStar retractor) is employed to aid in the dissection and mobilization of the bulbar urethra.
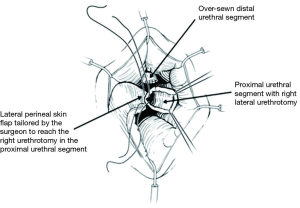
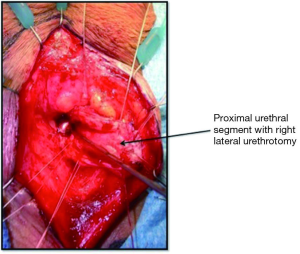
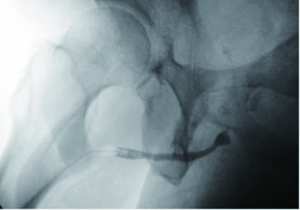
In order to maximize urethral length, the urethra is then amputated at the distal-most possible level, as determined by cystoscopy or intraoperative exam. The distal urethral stump is over-sewn using an absorbable, 3-0 chromic suture. A right-lateral urethrotomy is then made in the proximal urethral segment (Figure 3) and a 7-shaped skin flap is then custom tailored to fit the distance to the proximal urethrotomy (Figure 4). The skin of the perineum is matured to the urethra with interrupted, absorbable suture (Figure 4) (11). The urethrotomy is fashioned laterally until a 26 French bougie can be passed intravesically with ease.
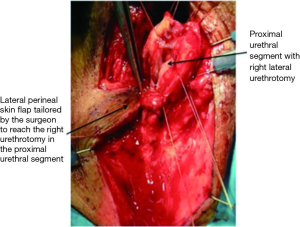
Care should be taken to ensure that the perineal skin flap is incised and developed with adequate thickness and rotated medially and proximally toward the proximal urethral stump. The right lateral incision of the “7” can be lengthened toward the anus to enable tension-free reapproximation to a deeper proximal urethral edge. Failure to perform the skin flap with adequate thickness may compromise the urethrocutaneous anastomosis due to flap necrosis from poor blood supply. To reduce unwanted tension, deep sutures can be placed to close any dead space between the urethral mucosa and the skin. The study authors leave a 16 French Foley catheter following 7-flap PU creation for a period of 7 postoperative days (Figure 5).
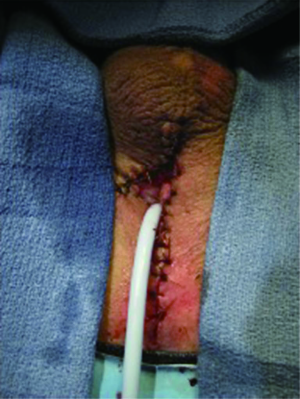
Outcomes and advantages of 7-flap PU
The authors now employ 7-flap PU as the standard procedure for proximal urethral urinary diversion. This surgical technique is easy to perform but more importantly, offers the surgeon added flexibility in tailoring urethrostomy creation based on intraoperative findings. Unlike the conventional approach to PU, the 7-flap PU can be performed if the surgeon decides intraoperatively that planned one-stage urethral repair will be unlikely to succeed or if temporary urethral diversion (e.g., a two-stage repair) is needed.
We first reported the use of 7-flap PU in ten patients with a 90% success rate. One patient in this original series required endoscopic dilation of his perineal neomeatus (11). In an expanded series of the initial report, we found no PU failures in an additional 12 patients undergoing this procedure. Reflecting a large number of patients with long urethra-to-skin distance, 63% of 7-flap PU patients in our series were obese (BMI ≥30). With a mean follow-up of 32 months, an overwhelming majority of patients (95%, n=21/22) had resolution of obstructive voiding without the need for any additional intervention.
The added ability to tailor the lateral based perineal skin flap intraoperatively is another advantage of the 7-flap PU over the conventional approach. Specifically, the inverted “Y” approach mandates the surgeon to determine the length of the scrotal skin flap prior to making the incision. Extension of the height of the “Y” is impossible anteriorly and risks involving the anal complex posteriorly. The 7-flap PU, however, can be initiated in a far more conservative manner and can be lengthened easily based on the calibration of the proximal urethral stump at the time of urethrotomy.
We have been able to perform 7-flap PU for a wide variety of indications in an array of patients. Patients with a long urethra-to-skin distance (e.g., obesity, Figure 6) undergo successful proximal urethral urinary diversion with our described 7-flap PU technique. The versatility offered by our easily adoptable technique prevents the need for ancillary maneuvers such as buccal mucosal grafting in complex patients with refractory stricture disease (12) (Figure 7). Additionally, unlike conventional PU, our technique avoids the need to perform a bilateral perineal skin flap down to the level of a fixed urethral plate using difficult-to-place apical stitches.

Our reported success of 95% with 7-flap PU compares favorably to patients undergoing conventional PU. Unlike traditional approaches, the 7-flap procedure does not involve the utilization of scrotal flap techniques. Although scrotal flaps are valuable adjuncts in urethral reconstructive surgery, scrotal tissue is often hair-bearing and may even occlude the urethral lumen in severe cases. We have yet to experience a case of 7-flap PU occlusion due to obstruction from perineal hair.
Conclusions
The PU is a valuable option for improving the quality of life in patients with debilitating urethral stricture. PU may be performed using a conventional approach or by a recently described, highly successful 7-flap technique. Although more customary approaches may be performed with acceptable success rates, contemporary reconstructive techniques have rendered PU performance applicable to highly refractory cases involving those patients with complex, advanced panurethral stricture disease or those that have failed prior urethroplasty. Compared to conventional PU, the authors believe 7-flap PU performance affords many advantages including a midline approach with the ability to tailor the incision intra-operatively as well as potentially improving outcomes in patients with perineal obesity and proximal bulbar strictures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blandy JP, Singh M, Tresidder GC. Urethroplasty by scrotal flap for long urethral strictures. Br J Urol 1968;40:261-7. [PubMed]
- Myers JB, Porten SP, McAninch JW. The outcomes of perineal urethrostomy with preservation of the dorsal urethral plate and urethral blood supply. Urology 2011;77:1223-7. [PubMed]
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol 2007;178:2268-76. [PubMed]
- Myers JB, McAninch JW. Perineal urethrostomy. BJU Int 2011;107:856-65. [PubMed]
- Palminteri E, Brandes SB, Djordjevic M. Urethral reconstruction in lichen sclerosus. Curr Opin Urol 2012;22:478-83. [PubMed]
- Blandy JP, Singh M, Notley RG, et al. The results and complications of scrotal-flap urethroplasty for stricture. Br J Urol 1971;43:52-7. [PubMed]
- Elliott SP, Eisenberg ML, McAninch JW. First-stage urethroplasty: utility in the modern era. Urology 2008;71:889-92. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. Clinical outcome and quality of life assessment in patients treated with perineal urethrostomy for anterior urethral stricture disease. J Urol 2009;182:548-57. [PubMed]
- Kulkarni S, Barbagli G, Kirpekar D, et al. Lichen sclerosus of the male genitalia and urethra: surgical options and results in a multicenter international experience with 215 patients. Eur Urol 2009;55:945-54. [PubMed]
- Peterson AC, Palminteri E, Lazzeri M, et al. Heroic measures may not always be justified in extensive urethral stricture due to lichen sclerosus (balanitis xerotica obliterans). Urology 2004;64:565-8. [PubMed]
- French D, Hudak SJ, Morey AF. The "7-flap" perineal urethrostomy. Urology 2011;77:1487-9. [PubMed]
- Kamat N. Perineal urethrostomy stenosis repair with buccal mucosa: description of technique and report of four cases. Urology 2008;72:1153-5. [PubMed]


