In situ urethroplasty after artificial urinary sphincter cuff erosion
Introduction
The artificial urinary sphincter (AUS) has evolved from its original description by Scott, over 40 years ago (1). Innovative treatments such as the male sling (2) and injectable agents (3) have been described; however, the AUS remains the gold standard for treatment of male stress urinary incontinence (SUI), with long-term safety and efficacy.
Short-term complications with AUS placement are rare; however large, long-term series have reported complication rates between 16-31% (4-8) with revision in up to 53-64% of cases (9,10). In particular, urethral cuff erosion remains a troublesome complication, occurring in 4.2-6.1% of patients (4-10). Traditional management of AUS cuff erosion consists of device removal with urinary diversion via Foley catheter placement or suprapubic cystostomy for several weeks (11). Further complicating the clinical course of an AUS cuff erosion is the urethral stricture formation during secondary healing of the urethral defect. Though not well reported, urethral stricture development in the AUS patient is a vexing problem requiring further manipulation such as dilation or incision/excision of the stricture prior to AUS replacement, if and when that ever becomes possible.
In situ urethroplasty (ISU) technique has recently been reported at the time of eroded cuff removal to prevent urethral stricture development (12). In this article we will review AUS cuff erosion and its risk factors, describe the ISU technique and discuss the benefits of ISU in these challenging patients.
Risk factors for AUS cuff erosion
Urethral erosion occurs in 4.2-6.1% of AUS cases, and although preventive measures such as delayed activation and overnight deactivation have been proposed over the past 20 years, AUS cuff erosion remains a troublesome complication (4-10,13). Multiple patient associated factors (smoking, coronary artery disease, obesity, hypertension, diabetes mellitus) have been linked to AUS complications (10,14). In particular, pelvic radiation, and its attendant obliterative endarteritis, has been shown to have a profound effect on cuff erosion rates (14-16). Likewise, AUS revision surgery has been associated with a higher cuff erosion rate, especially in those with a history of prior infection or erosion of the device (14-17).
Recently, the introduction of the smaller 3.5 cm cuff has provided incontinence surgeons a solution for small caliber urethras with positive initial results (18). It is possible, however, that cuff erosions may occur more commonly with use of the smaller 3.5 cm cuffs (16), especially in the high risk patient (19). Finally, neurogenic bladder patients have historically been shown to be at higher risk for AUS complications, including erosion (20), however more recent data would indicate that comparable outcomes are achievable in this population (8).
ISU technique
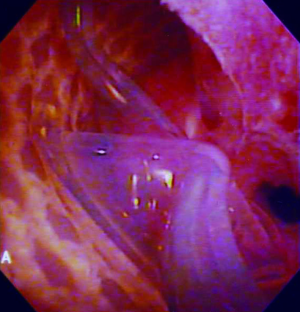
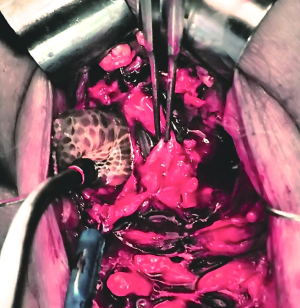
ISU is performed at the time of AUS device explantation. Figure 1 shows an endoscopic view of the eroded AUS cuff. After the cuff is removed, Foley catheter is inserted to facilitate reconstruction, and when necessary, urethroscopy with wire placement ensures accurate placement of a 14-French Council tip catheter (Figure 2). ISU is accomplished by reapproximating the urethral defect with full-thickness, interrupted 2-0 absorbable monofilament suture over the catheter (Figures 3 and 4). This abbreviated approach is performed without further mobilization of inflamed tissues within the already hostile operative field. The urethral Foley catheter is left in place for three weeks with voiding cystourethrogram (VCUG) imaging done at the time of removal.
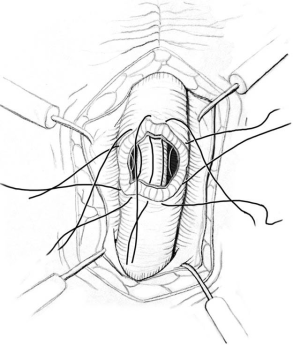
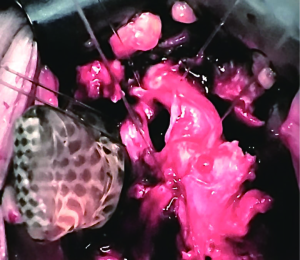
Results of ISU
Rozanski and colleagues examined the primary outcome of stricture formation after cuff erosion in two cohorts, those undergoing ISU at the time of cuff removal and those managed with traditional Foley catheter drainage after removal (12). The 26 patients in the two groups were well matched for age and comorbidities. ISU patients had a dramatically lower rate of stricture formation (5/13, 38%) compared to 11/13 (85%) in the traditional management group (P=0.047). Performing the ISU did not add significant time to the operative procedure, averaging an additional 8 minutes.
Patients undergoing an ISU received significantly fewer interim procedures prior to device replacement and had a higher chance of eventually receiving a subsequent AUS reimplantation surgery. ISU also decreased delay in AUS replacement, with an average interval of 9 months compared to 17 months. No patient who underwent secondary AUS implantation surgery experienced subsequent erosion over a mean follow-up interval of 24 months.
It was noted that most men who develop strictures following AUS cuff removal never have the device replaced, thus continuing to suffer from severe urinary incontinence. Re-establishing continence in these patients as soon as possible is of paramount importance because incontinent men experience significant reduction in their health-related quality of life (HRQL) (21,22). Incontinence, however mild, is associated with lifestyle modification, loss of work, social embarrassment, and an increased risk of hospitalization and nursing home admission (23,24). Treatment to restore continence should be as expeditious as possible to return them to normal activities. Resultant urethral strictures delay AUS replacement, subjecting patients to interval procedures, further magnifying the devastating sequelae of incontinence.
Advantages and limitations of ISU
ISU appears to be an expeditious, yet definitive repair, adding little operative time to AUS removal, with negligible complications related to ISU. Stricture formation with ISU as compared with traditional management with Foley catheter drainage alone is considerably lower. Finally, ISU patients usually had a new AUS implanted without further delay or additional procedures. Though quality of life has not specifically been compared, it is likely that ISU patients benefit by an earlier return to a better HRQL. The short follow-up in a small series of patients at a single institution, makes this technique less generalizable. Larger series, in a diverse population with similar results will gain this technique broader acceptance.
Conclusions
ISU offers promising results in the prevention of urethral stricture formation after AUS cuff erosion. The time to AUS reimplantation may be reduced compared to traditional management.
Acknowledgements
We thank Elsevier for the permission of reprinting Figure 3.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology 1973;1:252-9. [PubMed]
- Castle EP, Andrews PE, Itano N, et al. The male sling for post-prostatectomy incontinence: mean followup of 18 months. J Urol 2005;173:1657-60. [PubMed]
- Westney OL, Bevan-Thomas R, Palmer JL, et al. Transurethral collagen injections for male intrinsic sphincter deficiency: the University of Texas-Houston experience. J Urol 2005;174:994-7. [PubMed]
- Leibovich BC, Barrett DM. Use of the artificial urinary sphincter in men and women. World J Urol 1997;15:316-9. [PubMed]
- Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol 1998;159:1206-8. [PubMed]
- Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol 2001;166:1755-8. [PubMed]
- Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol 2005;173:1242-5. [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [PubMed]
- Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol 2000;164:702-6; discussion 706-7. [PubMed]
- Wang R, McGuire EJ, He C, et al. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology 2012;79:922-8. [PubMed]
- Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology 1996;48:906-8. [PubMed]
- Rozanski AT, Tausch TJ, Ramirez D, et al. Immediate urethral repair during explantation prevents stricture formation after artificial urinary sphincter cuff erosion. J Urol 2014;192:442-6. [PubMed]
- Motley RC, Barrett DM. Artificial urinary sphincter cuff erosion. Experience with reimplantation in 38 patients. Urology 1990;35:215-8. [PubMed]
- Raj GV, Peterson AC, Webster GD. Outcomes following erosions of the artificial urinary sphincter. J Urol 2006;175:2186-90; discussion 2190. [PubMed]
- Lai HH, Boone TB. Complex artificial urinary sphincter revision and reimplantation cases--how do they fare compared to virgin cases? J Urol 2012;187:951-5. [PubMed]
- Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. [PubMed]
- Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol 2014;191:734-8. [PubMed]
- Hudak SJ, Morey AF. Impact of 3.5 cm artificial urinary sphincter cuff on primary and revision surgery for male stress urinary incontinence. J Urol 2011;186:1962-6. [PubMed]
- Simhan J, Morey AF, Singla N, et al. 3.5 cm Artificial Urinary Sphincter Cuff Erosion Occurs Predominantly in Irradiated Patients. J Urol 2015;193:593-7. [PubMed]
- Murphy S, Rea D, O'Mahony J, et al. A comparison of the functional durability of the AMS 800 artificial urinary sphincter between cases with and without an underlying neurogenic aetiology. Ir J Med Sci 2003;172:136-8. [PubMed]
- Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CaPSURE. J Urol 2004;171:703-7; discussion 707-8. [PubMed]
- Arredondo SA, Elkin EP, Marr PL, et al. Impact of comorbidity on health-related quality of life in men undergoing radical prostatectomy: data from CaPSURE. Urology 2006;67:559-65. [PubMed]
- Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing 1997;26:367-74. [PubMed]
- Cooperberg MR, Master VA, Carroll PR. Health related quality of life significance of single pad urinary incontinence following radical prostatectomy. J Urol 2003;170:512-5. [PubMed]