Penile rehabilitation after radical prostatectomy: does it work?
Introduction
Prostate cancer (PCa) represents one of the most frequently diagnosed malignancies in the United States and Europe (1). Radical prostatectomy (RP) is one of the most commonly adopted therapeutic options in patients with clinically localized PCa (2). Although this surgical approach is associated with excellent long-term oncologic results (3-5), the risk of short- and long-term adverse events is not negligible (5). Particularly, urinary incontinence (UI) and erectile dysfunction (ED) represent long-term sequelae observed in a non-negligible proportion of patients treated with RP. Of note, these side effects are associated with a profound detrimental impact on patient health-related quality of life.
A number of studies reported satisfactory urinary continence recovery rates after surgery (5-13). However, the postoperative recovery of erectile function (EF) still represents a major challenge for patients and physicians. When considering the risk of ED after surgery, several factors should be considered. First, preoperative patient characteristics play a major role on the subsequent probability of recovering EF after surgery, where younger and healthier individuals have substantially higher recovery rates as compared to their older and sicker counterparts (9,13-18). Second, preoperative EF represents a significant predictor of the subsequent risk of ED after surgery (14-16,19). Indeed, the probability of achieving satisfactory erections after surgery is extremely low in patients with severe ED as measured by the International Index of Erectile Function (IIEF) (14-16,19,20). Moreover, patients with higher preoperative IIEF might represent individuals more motivated to achieve satisfactory erectile and sexual function after surgery (21). Finally, the surgical technique and surgeon experience have a substantial impact on the probability of ED after surgery (20,22-28). In this context, the knowledge of the surgical anatomy, together with continuous refinements in the surgical approaches and the introduction of minimally invasive surgery might have resulted in improved potency outcomes after surgery (23-31). For example, surgical approaches aimed at preserving the neurovascular bundles deputed to erections have been developed over the last decades (23,24,26). Moreover, the better visualization of the surgical field, as well as lower intraoperative bleeding and more precise excision associated with robot-assisted RP might result into improved functional outcomes at long-term follow-up (25).
Although accurate patient selection and improvements in the surgical technique might minimize the risk of ED after surgery, the removal of the prostate leads to the temporary loss of erections. This would, in turn, result into reduced oxygenation, pro-apoptotic, and pro-fibrotic changes in the corpora cavernosa that would finally result in postoperative ED (31-34). In this context, penile rehabilitation after RP has been proposed as a therapeutic option in order to break this vicious circle, promoting erectile tissue preservation and preventing pro-apoptotic and pro-fibrotic alterations in the corpora cavernosa (31,32).
This review aims at analyzing the rationale of penile rehabilitation after RP in patients with clinically localized PCa. Moreover, we sought to comprehensively evaluate basic science and clinical evidences supporting the adoption of penile rehabilitation after RP.
Evidence acquisition
A literature review was performed in September 2014 using the Medline, Embase, and Web of Science databases. The search strategy included the terms prostate cancer, penile rehabilitation, sexual function, radical prostatectomy, erectile dysfunction, phosphodiesterase type-5 inhibitors, alone or in combination. We limited our search to large population-based retrospective studies and prospective investigations published between January 2005 and September 2014. Cited references from selected articles and from review articles retrieved in our search were also used to identify manuscripts that were not included in the initial search.
Records were considered relevant to this review if they included patients diagnosed with clinically localized PCa. Only studies including patients treated with RP were evaluated. Only studies assessing EF after RP according to validated tools were evaluated. Results coming from prospective multi-institutional trials were preferred over retrospective single-center studies. Case reports, editorials, and letters were excluded during the review process. Additionally, unpublished data or meeting abstracts were excluded because information that is needed to correctly assess the study quality is usually not available in abstracts.
The primary outcome was the recovery of EF after surgery. The definition of EF recovery was the one used by individual studies.
The articles that provided the highest level of evidence were evaluated and selected with the consensus of all the author of this manuscript. A total of 81 articles were reviewed (Figure 1).
Evidence synthesis
The definition of penile rehabilitation and its rationale
The pioneering work of Montorsi et al. (35) firstly introduced the concept of penile rehabilitation after RP in the year 1997. Nowadays, penile rehabilitation is defined as the use of any intervention or combination with the goal not only to achieve erections sufficient for satisfactory sexual intercourses, but also to return EF to preoperative levels (31). The rationale of penile rehabilitation is strongly linked to the pathophysiology of ED after RP. In healthy men, during erections the penis oxygenation rises from 35-40 to 75-100 mmHg and there is a balance between the flaccid status and erect status (36). Thus, erectile tissues oxygenation is preserved as long as men obtain erections regularly. In patients undergoing RP, neuropraxia occurs due to direct trauma, inflammation, heating, and ischemia affecting the cavernous nerves, even in men treated with nerve-sparing procedures (32,37,38). The chronic absence of erections related to cavernous nerves neuropraxia after surgery would result in a state of persisting flaccidity. This, in turn, would lead to fibrogenic cytokine production (e.g., increased expression of TGF-β1, ET-1, NGF, and HIF-1α) and to structural changes in erectile tissues (36,39-41), which might finally result into smooth muscle apoptosis and fibrosis (42). The overexpression of fibrotic tissue would eventually impair the corpora cavernosa elasticity compressive action on subtunical venules, ultimately resulting in postoperative ED (32).
The concept of penile rehabilitation is based on the implementation of therapeutic protocols aimed at improving cavernosal oxygenation, preserving endothelial structure, and finally preventing smooth muscle structural changes (31,36). Nowadays, the most commonly adopted approaches for penile rehabilitation after RP in PCa patients are represented by the administration of PDE5-Is, intracorporeal injection therapy, vacuum erection devices (VED), and the combination of these treatments (31,32).
Phosphodiesterase type-5 inhibitors (PDE5-Is)
The administration of PDE5-Is represents the most commonly performed type of penile rehabilitation after RP, where up to 87% of the participants adopted this treatment strategy (43,44).
Although clinical studies reported conflicting results with regards to the efficacy of rehabilitation protocols based on the administration of PDE5-Is (45-51), preclinical data support the beneficial effects of these molecules (52-64). Indeed, several investigations demonstrated that the chronic administration of PDE5-Is to rats undergoing cavernous nerve injury might decrease erectile tissue fibrosis and apoptosis of smooth muscle (52,53,61-64). In this context, Sildenafil administration has been found to affect the expression of several genes involved in smooth muscle preservation and to reduce oxidative stress (32,56,58). Additionally, the administration of PDE5-Is has been proposed to prevent the degeneration of nervous tissue and stimulate neuroregeneration (61,65). Indeed, an increased amount of nerves has been observed after cavernous nerve injury in rats treated with sildenafil compared to their counterparts left untreated (61). Finally, PDE5-Is might also have a role in endothelial cell preservation, conserving platelet endothelial cell adhesion molecule-1 (CD31) and endothelial Nitric Oxid Synthase (eNOS) expression (54,66). On the other hand, human studies evaluating the morphologic changes to cavernous tissue after the administration of Sildenafil in patients treated with RP reported conflicting results, where neither elastic fibers nor connective tissue content substantially changed compared to preoperative levels (67,68). However, these investigations are limited by the small number of patients evaluated, by heterogeneity in the surgical technique, and by the lack of a control group.
Taken together, the results of these preclinical studies raised the hypothesis that early administration of PDE5-Is might improve EF recovery after RP and inspired the design of several prospective trials. Table 1 depicts the characteristics and results of studies evaluating the effectiveness of penile rehabilitation protocols based on the administration of four different PDE5-Is (Figure 2).

Full table
In their pioneering trial, Padma-Nathan et al. (51) randomized 76 patients treated with nerve-sparing RP to sildenafil or placebo nightly for 36 weeks followed by a 8-week drug-free period. Interestingly the authors demonstrated that the return to baseline EF was more marked for men treated with PDE5-Is compared to their counterparts receiving placebo. Moreover, the mean Erectile Function domain of the IIEF (IIEF-EF) was substantially higher in the sildenafil group. Finally, nightly administration of PDE5-Is markedly improved nocturnal penile tumescence and rigidity in patients treated with sildenafil (69). Although this study reported encouraging results and introduced for the first time the concept of penile rehabilitation using PDE5-Is, enrolment ceased early owing to interim analyses showing a lower response rate than expected. Moreover, the lack of a group receiving on-demand dosing limits the applicability of these findings. Under this light, it is worth reporting that a recent randomized trial evaluating patients treated with bilateral nerve-sparing robot-assisted RP failed to show statistically significant differences between patients receiving sildenafil on-demand or nightly at 13-month follow-up (45). However, these results are limited by the small number of patients evaluated (n=100), as well as by the lack of a placebo group and the relatively short follow-up period.
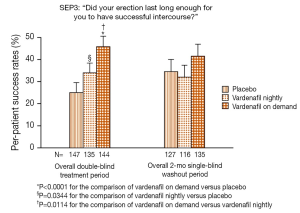
A well-performed randomized controlled trial evaluated the efficacy of penile rehabilitation using vardenafil (50). During a 9-month double-blind period, patients were randomized to placebo, nightly 10 mg vardenafil, and on-demand 10 mg vardenafil. Interestingly, on-demand vardenafil treatment resulted in significantly greater IIEF-EF scores and higher response rates to the Sexual Encounter Profile question 3 [(SEP3); “Did your erection last long enough for you to have successful intercourse?”] than placebo over the entire double-blind treatment period. Patients were then evaluated after an additional 2-month washout period. At this time-point EF recovery was not improved by nightly or on-demand vardenafil compared to placebo (Figure 3). Similarly, after a 2-month open-label period no statistically significant differences were observed among treatment groups with respect to IIEF-EF score or SEP3 success rates. Of note, the superiority of the on-demand dosing during the double-blind treatment period might be related to the pharmacokinetic of vardenafil, its onset of action, and the half-life of this drug (70,71). Indeed, patients receiving the drug on-demand might have had the full effect of the treatment when needed, while those in the nightly group had an effect so far as their sexual activity coincided with the administration of vardenafil (32). On the other hand, difficulties to reach a steady state with a single daily administration might limit the efficacy of chronic vardenafil dosing in terms of preservation of erectile tissue after surgery.
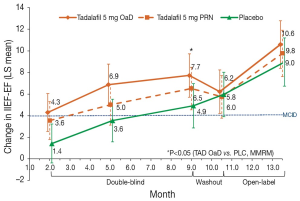
When evaluating the efficacy of tadalafil in the penile rehabilitation setting, a randomized controlled study failed to show an improvement in penile length and EF recovery after the administration of 20 mg tadalafil 3 times a week for 6 months (72). However, the small number of patients, short follow-up, and excellent postoperative EF-recovery rates in the placebo group raise some concerns regarding the generalizability of these findings. More recently, a larger study by Montorsi et al. (48) evaluated the efficacy tadalafil compared to placebo in the recovery of EF after nerve-sparing RP. Patients were randomized to receive 5 mg tadalafil once daily, 20 mg tadalafil on-demand, or placebo. At the end of the double-blind period (9 months), the IIEF-EF score improvement exceeded the minimally clinically important difference in both tadalafil groups. However, only patients treated with tadalafil once daily had a statistically significant difference in the change in IIEF-EF compared to placebo at this time point. Although the IIEF-EF and SEP-3 improved also during the open-label phase of the study exceeding the minimal clinically important difference for all the groups, no differences were observed between patients treated with tadalafil and placebo after open-label treatment (Figure 4). When considering the SEP-3 question, only patients receiving tadalafil once daily had a significant improvement compared to their counterparts receiving placebo at the end of the double-blind period and after open-label treatment. However, no significant differences were observed after 6 weeks drug-free washout. Finally, significantly less shrinkage of penile length was observed in the tadalafil once daily group as compared to placebo at the end of the double-blind period. Concluding, the administration of Tadalafil once daily seems to be to be more effective than placebo and on-demand dosing in patients with ED following nerve-sparing RP. Although these results were not maintained at the end of the washout period, the administration of tadalafil once daily might have contributed to the preservation of erectile tissue, preventing alterations to the cavernosal integrity as a sequel of neuropraxia typical of patients undergoing RP (36,39-41). A subanalysis performed in the same cohort by Moncada et al. (73) recently demonstrated that the administration of tadalafil once daily significantly shortened the time to EF recovery during the 9-month double-blind treatment period. Additionally, at Cox regression analyses patients treated with tadalafil once daily had substantially 1.9-fold higher probability of recovering EF after surgery as compared to their counterparts treated with placebo. However, this did not hold true for patients receiving tadalafil on-demand. Of note, the pharmacokinetic profile of tadalafil and his half-life might confer to this molecule the best profile for its use in the rehabilitation setting compared to other PDE5-Is such as sildenafil and vardenafil (70,71,74).
More recently, the efficacy of avanafil in the recovery of EF after RP has also been tested. A randomized trial by Mulhall et al. (75) demonstrated that patients receiving 100 or 200 mg avanafil on-demand had substantially higher IIEF-EF and SEP-3 response rates compared to placebo at 12-week follow-up after bilateral nerve-sparing RP. However, the lack of a group of patients receiving avanafil daily precludes a proper generalization of these findings in the penile rehabilitation context.
Taken together, these observations demonstrate that penile rehabilitation might improve postoperative EF in patients treated with nerve-sparing RP for clinically localized PCa (45,48,50,51,69,75). Nonetheless, while basic science studies support the efficacy of PDE5-Is in the preservation of erectile tissues after RP, clinical investigations report contrasting findings. Although chronic administration of tadalafil might represent the best choice in order to prevent alterations to cavernous tissues typical of patients undergoing RP (48,74), the superiority of this treatment over on-demand administration of PDE5-Is is still debated. Nowadays, none of the available randomized controlled trials definitively demonstrated the superiority of daily administration of PDE5-Is compared to the on-demand dosing. Moreover, the beneficial effects of penile rehabilitation protocols using PDE5-Is compared to placebo do not seem to be maintained after the washout period. Nonetheless, basic science and clinical data support the idea that rehabilitation treatments with PDE5-Is are undoubtedly better than leaving the cavernous tissues untreated after nerve-sparing surgery (49,76,77). Further well-designed and well-performed studies with proper patient selection are needed to finally address this issue (74). Indeed, the main limitations of currently available prospective randomized-controlled trials assessing the efficacy of PDE5-Is in the penile rehabilitation setting reside in the relatively short follow-up period, treatment duration and timing of drug administration, type of PDE5-Is chosen, and stringent selection criteria. Patients receiving PDE5-Is in a penile rehabilitation setting should begin treatment as soon as possible and as close to surgery as possible (31,32,49,78,79). Therefore, future randomized trials should include patients treated as early as the removal of the catheter or during the very first months after surgery (80). Moreover, a recent study demonstrated that a 9-month double-blind treatment period was too short to achieve satisfactory EF recovery in the majority of the patients enrolled (73). Therefore, longer treatments could be considered in future studies. Additionally, tadalafil might have the best profile for its use in the penile rehabilitation setting due to his long half-life (70,71,74). Therefore, future studies might focus on this molecule. Finally, patient selection might play a crucial role in the context of prospective randomized-controlled trials assessing the role of PDE5-Is on EF recovery after surgery. Indeed, the inclusion of best candidates for EF recovery (i.e., younger and healthier patients with lower probability of ED after surgery) might limit the effects of PDE5-Is administration (14-16,74). On the other hand, the maximal effect of chronic use of PDE5-Is might be achieved in patients with less favorable preoperative characteristics (14-16,74). Therefore, future studies should adopt less stringent criteria to evaluate the efficacy of PDE5-Is on EF recovery in these patients.
It should also be noted that a recent study demonstrated that patients receiving PDE5-Is after surgery might be more likely to experience biochemical recurrence compared to their counterparts left untreated (81). However, these data come from one single study and are not fully supported by preclinical evidences (81-85). Moreover, the lack of details on the type of PDE5-Is used, as well as dosing and duration of treatment strongly limits the applicability of these findings. Further well-designed studies are needed to better address this issue.
Intracavernosal injections
Montorsi et al. (35) in the year 1997 performed a pioneering study aimed at assessing the efficacy of intracavernosal injections of alprostadil for the recovery of spontaneous erections after nerve-sparing RP. Although the study was partially limited by the relatively small number of patients evaluated, early administration of alprostadil significantly increased the recovery rates of EF after surgery. From a biological standpoint, the administration of alprostadil might result into erections, which improve cavernosal oxygenation and penile stretch, finally resulting into a protective effect on erectile tissues (31). Of note, other non-randomized studies supported the efficacy of intracavernosal injections in the recovery of EF after surgery, even after initial administration of sildenafil (86-88). However, when considering this approach, high patient motivation and adherence to protocol are required to increase the compliance to this treatment modality and minimize the dropout rates (21,86). Concluding, intracavernosal injections might be effective in men who have tried oral agents and their condition has failed to respond. Despite this, evidences supporting the efficacy of intracavernosal injections in a rehabilitation setting are still scarce. Additionally, patient compliance is still sub-optimal. Taken together, these aspects prevent clinicians to routinely recommend the adoption of this treatment modality in penile rehabilitation after RP (49).
Vacuum devices
Vacuum devices create a vacuum around the penis. This results into a transient increase in arterial flow and oxygen supply to the erectile tissues (31,32,36,89). Preclinical studies in rats undergoing cavernous nerve injury demonstrated that VED therapy might facilitate EF recovery after surgery acting both on the preservation of smooth muscle and endothelial integrity via anti-hypoxia, anti-apoptosis, and antifibrotic mechanisms (90). These observations were only in part confirmed by randomized studies comparing EF recovery in patients receiving VED or placebo after nerve-sparing RP (91-93). In their pioneering study, Raina et al. (92) evaluated 109 patients who developed ED after nerve-sparing surgery. The authors demonstrated that early use of VED facilitated early sexual intercourses, sexual satisfaction, and early return of natural erections sufficient for vaginal penetration. More recently, Basal et al. (93) randomized more than 200 patients treated with robotic-assisted RP to VED, PDE5-Is alone, VED and PDE5-Is, or placebo. Of note, the authors demonstrated that only PDE5-Is or the combination of PDE5-Is and VED had a beneficial effect on the recovery of EF after surgery. On the other hand, VED alone failed to show a beneficial effect with regards to postoperative EF recovery. These results were limited by the low number of patients and by the heterogeneity in preoperative characteristics, where a non-negligible proportion of these individuals had ED before surgery.
Concluding, VED alone or in association with PDE5-Is might represent a treatment option for penile rehabilitation in patients treated with nerve-sparing RP. However, evidences supporting the efficacy of this approach are scarce. Moreover, large well-designed and performed prospective randomized studies assessing the superiority of this approach compared to PDE5-Is and/or intracavernous injections are still lacking. Lastly, available studies do not support a long-term effect of this approach on postoperative EF recovery. As a consequence, VED is not recommended by clinical guidelines for the recovery of EF after surgery. Despite this, VED might represent a treatment option in selected patients.
Although we comprehensively reviewed the currently available literature regarding the role of penile rehabilitation after RP, our manuscript does not represent a systematic review and/or a meta-analysis. Therefore, it cannot provide the same level of evidence of these types of articles. Meta-analyses of currently available prospective randomized trials evaluating the role of PDE5-Is, intracavernosal injections, and vacuum devices are needed to definitively assess the role these therapies in the penile rehabilitation setting.
Conclusions
Currently available penile rehabilitation protocols are based on the administration of PDE5-Is, intracavernosal injections, and VED. Basic science evidences support the rationale of penile rehabilitation after nerve-sparing RP in patients with clinically localized PCa. However, clinical trials report conflicting results regarding the potential benefit of penile rehabilitation in terms of EF recovery and erectile tissue preservation after nerve-sparing RP. Although rehabilitation, set as early as possible, seems to be better than leaving the erectile tissues unassisted, which is the optimal rehabilitation program still represents a matter of debate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 2013;309:2587-95. [PubMed]
- Schiavina R, Borghesi M, Dababneh H, et al. Survival, Continence and Potency (SCP) recovery after radical retropubic prostatectomy: a long-term combined evaluation of surgical outcomes. Eur J Surg Oncol 2014;40:1716-23. [PubMed]
- Mullins JK, Feng Z, Trock BJ, et al. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol 2012;188:2219-24. [PubMed]
- Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol 2012;61:664-75. [PubMed]
- Suardi N, Moschini M, Gallina A, et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int 2013;111:717-22. [PubMed]
- Gandaglia G, Suardi N, Gallina A, et al. Preoperative erectile function represents a significant predictor of postoperative urinary continence recovery in patients treated with bilateral nerve sparing radical prostatectomy. J Urol 2012;187:569-74. [PubMed]
- Gandaglia G, Albersen M, Suardi N, et al. Postoperative phosphodiesterase type 5 inhibitor administration increases the rate of urinary continence recovery after bilateral nerve-sparing radical prostatectomy. Int J Urol 2013;20:413-9. [PubMed]
- Gandaglia G, Suardi N, Gallina A, et al. How to optimize patient selection for robot-assisted radical prostatectomy: functional outcome analyses from a tertiary referral center. J Endourol 2014;28:792-800. [PubMed]
- Prabhu V, Sivarajan G, Taksler GB, et al. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur Urol 2014;65:52-7. [PubMed]
- Srivastava A, Chopra S, Pham A, et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur Urol 2013;63:438-44. [PubMed]
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405-17. [PubMed]
- Abdollah F, Sun M, Suardi N, et al. Prediction of functional outcomes after nerve-sparing radical prostatectomy: results of conditional survival analyses. Eur Urol 2012;62:42-52. [PubMed]
- Briganti A, Di Trapani E, Abdollah F, et al. Choosing the best candidates for penile rehabilitation after bilateral nerve-sparing radical prostatectomy. J Sex Med 2012;9:608-17. [PubMed]
- Briganti A, Gallina A, Suardi N, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med 2010;7:2521-31. [PubMed]
- Briganti A, Capitanio U, Chun FK, et al. Prediction of sexual function after radical prostatectomy. Cancer 2009;115:3150-9. [PubMed]
- Gallina A, Ferrari M, Suardi N, et al. Erectile function outcome after bilateral nerve sparing radical prostatectomy: which patients may be left untreated? J Sex Med 2012;9:903-8. [PubMed]
- Teloken PE, Nelson CJ, Karellas M, et al. Defining the impact of vascular risk factors on erectile function recovery after radical prostatectomy. BJU Int 2013;111:653-7. [PubMed]
- Gacci M, Carini M, Simonato A, et al. Factors predicting continence recovery 1 month after radical prostatectomy: results of a multicenter survey. Int J Urol 2011;18:700-8. [PubMed]
- Harris CR, Punnen S, Carroll PR. Men with low preoperative sexual function may benefit from nerve sparing radical prostatectomy. J Urol 2013;190:981-6. [PubMed]
- Gandaglia G, Gallina A, Suardi N, et al. Preoperative erectile function is the only predictor of the use of a high number of phosphodiesterase type-5 inhibitors after bilateral nerve-sparing radical prostatectomy. Int J Impot Res 2014;26:201-4. [PubMed]
- Gandaglia G, Suardi N, Gallina A, et al. Extended pelvic lymph node dissection does not affect erectile function recovery in patients treated with bilateral nerve-sparing radical prostatectomy. J Sex Med 2012;9:2187-94. [PubMed]
- Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol 1982;128:492-7. [PubMed]
- Walsh PC. The discovery of the cavernous nerves and development of nerve sparing radical retropubic prostatectomy. J Urol 2007;177:1632-5. [PubMed]
- Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012;62:418-30. [PubMed]
- Schatloff O, Chauhan S, Sivaraman A, et al. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol 2012;61:796-802. [PubMed]
- Alemozaffar M, Duclos A, Hevelone ND, et al. Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. Eur Urol 2012;61:1222-8. [PubMed]
- Graefen M, Beyer B, Schlomm T. Outcome of radical prostatectomy: is it the approach or the surgical expertise? Eur Urol 2014;66:457-8. [PubMed]
- Moskovic DJ, Alphs H, Nelson CJ, et al. Subjective characterization of nerve sparing predicts recovery of erectile function after radical prostatectomy: defining the utility of a nerve sparing grading system. J Sex Med 2011;8:255-60. [PubMed]
- Gandaglia G, Sammon JD, Chang SL, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol 2014;32:1419-26. [PubMed]
- Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med 2013;10:195-203. [PubMed]
- Fode M, Ohl DA, Ralph D, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int 2013;112:998-1008. [PubMed]
- Leungwattanakij S, Bivalacqua TJ, Usta MF, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl 2003;24:239-45. [PubMed]
- Iacono F, Giannella R, Somma P, et al. Histological alterations in cavernous tissue after radical prostatectomy. J Urol 2005;173:1673-6. [PubMed]
- Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol 1997;158:1408-10. [PubMed]
- Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol 2008;18:613-20. [PubMed]
- Masterson TA, Serio AM, Mulhall JP, et al. Modified technique for neurovascular bundle preservation during radical prostatectomy: association between technique and recovery of erectile function. BJU Int 2008;101:1217-22. [PubMed]
- Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy. Urology 2003;61:491-7. [PubMed]
- Moreland RB, Albadawi H, Bratton C, et al. O2-dependent prostanoid synthesis activates functional PGE receptors on corpus cavernosum smooth muscle. Am J Physiol Heart Circ Physiol 2001;281:H552-8. [PubMed]
- Müller A, Tal R, Donohue JF, et al. The effect of hyperbaric oxygen therapy on erectile function recovery in a rat cavernous nerve injury model. J Sex Med 2008;5:562-70. [PubMed]
- Lee CH, Kim HS, Goo MJ, et al. Chronic administration of udenafil, a selective phosphodiesterase type 5 inhibitor, promotes erectile function recovery in an animal model of bilateral cavernous nerve crush injury. J Sex Med 2011;8:1330-40. [PubMed]
- Moreland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res 1998;10:113-20. [PubMed]
- Teloken P, Mesquita G, Montorsi F, et al. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the international society for sexual medicine practitioners. J Sex Med 2009;6:2032-8. [PubMed]
- Tal R, Teloken P, Mulhall JP. Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med 2011;8:2370-6. [PubMed]
- Pavlovich CP, Levinson AW, Su LM, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int 2013;112:844-51. [PubMed]
- McCullough AR, Hellstrom WG, Wang R, et al. Recovery of erectile function after nerve sparing radical prostatectomy and penile rehabilitation with nightly intraurethral alprostadil versus sildenafil citrate. J Urol 2010;183:2451-6. [PubMed]
- Bannowsky A, Schulze H, van der Horst C, et al. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. BJU Int 2008;101:1279-83. [PubMed]
- Montorsi F, Brock G, Stolzenburg JU, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). Eur Urol 2014;65:587-96. [PubMed]
- Salonia A, Burnett AL, Graefen M, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol 2012;62:273-86. [PubMed]
- Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol 2008;54:924-31. [PubMed]
- Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res 2008;20:479-86. [PubMed]
- Ferrini MG, Davila HH, Kovanecz I, et al. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology 2006;68:429-35. [PubMed]
- Vignozzi L, Filippi S, Morelli A, et al. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med 2006;3:419-31. [PubMed]
- Hatzimouratidis K, Burnett AL, Hatzichristou D, et al. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol 2009;55:334-47. [PubMed]
- Ferrini MG, Kovanecz I, Sanchez S, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod 2007;76:915-23. [PubMed]
- Lagoda G, Jin L, Lehrfeld TJ, et al. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med 2007;4:908-16. [PubMed]
- Hlaing SM, Garcia LA, Kovanecz I, et al. Sildenafil promotes neuroprotection of the pelvic ganglia neurones after bilateral cavernosal nerve resection in the rat. BJU Int 2013;111:159-70. [PubMed]
- Sirad F, Hlaing S, Kovanecz I, et al. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med 2011;8:1048-60. [PubMed]
- Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int 2008;101:203-10. [PubMed]
- Kovanecz I, Rambhatla A, Ferrini M, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res 2008;20:202-12. [PubMed]
- Mulhall JP, Müller A, Donohue JF, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med 2008;5:1126-36. [PubMed]
- Lysiak JJ, Yang SK, Klausner AP, et al. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol 2008;179:779-85. [PubMed]
- Ferrini MG, Kovanecz I, Sanchez S, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med 2009;6:415-28. [PubMed]
- Özden E, Öztürk B, Koşan M, et al. Effect of sildenafil citrate on penile weight and physiology of cavernous smooth muscle in a post-radical prostatectomy model of erectile dysfunction in rats. Urology 2011;77:761.e1-7.
- Zhang R, Wang Y, Zhang L, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke 2002;33:2675-80. [PubMed]
- Mazzola C, Mulhall JP. Penile rehabilitation after prostate cancer treatment: outcomes and practical algorithm. Urol Clin North Am 2011;38:105-18. [PubMed]
- Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol 2004;171:771-4. [PubMed]
- Iacono F, Prezioso D, Somma P, et al. Histopathologically proven prevention of post-prostatectomy cavernosal fibrosis with sildenafil. Urol Int 2008;80:249-52. [PubMed]
- McCullough AR, Levine LA, Padma-Nathan H. Return of nocturnal erections and erectile function after bilateral nerve-sparing radical prostatectomy in men treated nightly with sildenafil citrate: subanalysis of a longitudinal randomized double-blind placebo-controlled trial. J Sex Med 2008;5:476-84. [PubMed]
- Smith WB 2nd, McCaslin IR, Gokce A, et al. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract 2013;67:768-80. [PubMed]
- Montorsi F, Padma-Nathan H, Buvat J, et al. Earliest time to onset of action leading to successful intercourse with vardenafil determined in an at-home setting: a randomized, double-blind, placebo-controlled trial. J Sex Med 2004;1:168-78. [PubMed]
- Aydogdu O, Gokce MI, Burgu B, et al. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. Int Braz J Urol 2011;37:336-44; discussion 344-6. [PubMed]
- Moncada I, de Bethencourt FR, Lledó-García E, et al. Effects of tadalafil once daily or on demand versus placebo on time to recovery of erectile function in patients after bilateral nerve-sparing radical prostatectomy. World J Urol 2014. [Epub ahead of print]. [PubMed]
- Castiglione F, Nini A, Briganti A. Penile rehabilitation with phosphodiesterase type 5 inhibitors after nerve-sparing radical prostatectomy: are we targeting the right patients? Eur Urol 2014;65:673-4. [PubMed]
- Mulhall JP, Burnett AL, Wang R, et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol 2013;189:2229-36. [PubMed]
- Kaiho Y, Yamashita S, Arai Y. Optimization of sexual function outcome after radical prostatectomy using phosphodiesterase type 5 inhibitors. Int J Urol 2013;20:285-9. [PubMed]
- Mulhall JP, Parker M, Waters BW, et al. The timing of penile rehabilitation after bilateral nerve-sparing radical prostatectomy affects the recovery of erectile function. BJU Int 2010;105:37-41. [PubMed]
- Mulhall JP, Bella AJ, Briganti A, et al. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med 2010;7:1687-98. [PubMed]
- Park DL, Aron M, Rewcastle JC, et al. A model for managing erectile dysfunction following prostate cancer treatment. Curr Opin Urol 2013;23:129-34. [PubMed]
- Serino A, Castagna G, Capogrosso P, et al. Prevention and management of post prostatectomy erectile dysfunction. Transl Androl Urol 2014. [Epub ahead of print].
- Michl U, Molfenter F, Graefen M, et al. Use of phosphodiesterase type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy. J Urol 2015;193:479-83. [PubMed]
- Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691-702. [PubMed]
- Booth L, Roberts JL, Cruickshanks N, et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol 2014;85:408-19. [PubMed]
- Hirsh L, Dantes A, Suh BS, et al. Phosphodiesterase inhibitors as anti-cancer drugs. Biochem Pharmacol 2004;68:981-8. [PubMed]
- Savai R, Pullamsetti SS, Banat GA, et al. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs 2010;19:117-31. [PubMed]
- Polito M, d'Anzeo G, Conti A, et al. Erectile rehabilitation with intracavernous alprostadil after radical prostatectomy: refusal and dropout rates. BJU Int 2012;110:E954-7. [PubMed]
- Mulhall J, Land S, Parker M, et al. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med 2005;2:532-40; discussion 540-2. [PubMed]
- Nandipati K, Raina R, Agarwal A, et al. Early combination therapy: intracavernosal injections and sildenafil following radical prostatectomy increases sexual activity and the return of natural erections. Int J Impot Res 2006;18:446-51. [PubMed]
- Broderick GA, McGahan JP, Stone AR, et al. The hemodynamics of vacuum constriction erections: assessment by color Doppler ultrasound. J Urol 1992;147:57-61. [PubMed]
- Yuan J, Lin H, Li P, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol 2010;58:773-80. [PubMed]
- Köhler TS, Pedro R, Hendlin K, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int 2007;100:858-62. [PubMed]
- Raina R, Agarwal A, Ausmundson S, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res 2006;18:77-81. [PubMed]
- Basal S, Wambi C, Acikel C, et al. Optimal strategy for penile rehabilitation after robot-assisted radical prostatectomy based on preoperative erectile function. BJU Int 2013;111:658-65. [PubMed]