Cytoreductive surgery in the era of targeted molecular therapy
Introduction
Renal cell carcinoma (RCC) is a common malignancy that comprises approximately 3.9% of new cancers with up to 25% of RCC patients demonstrating evidence of systemic metastases at diagnosis (1). Historically, patients with metastatic RCC (mRCC) have poor prognosis with a 2-year survival of 10-20% (2). Over the last two decades, systemic management of metastatic RCC has significantly changed with increased understanding of the molecular biology of RCC. Agents such as sunitinib, sorafenib and temsirolimus, everolimus, and axitinib specifically target relevant biological pathways including vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR), respectively and have revolutionized the treatment of advanced RCC and replacing immunotherapy as first line therapy (3).
Despite such advances in the medical treatment of mRCC, cytoreductive surgery continues to play a dominant role in managing patients with advanced disease. Evidence for surgery primarily originates from randomized control trials from the immunotherapy era. Similar prospective studies assessing the efficacy of surgery and newer targeted agents are still under accrual and are not yet available for scrutiny (4). This review examines the current evidence and controversies of surgical intervention in the new era of targeted therapy for mRCC.
The SWOG and EORTC trials—evidence for cytoreductive nephrectomy (CN)
Before the advent of targeted therapy, CN in conjunction with postsurgical immunotherapy for metastatic RCC was the standard of care. The use of immuno therapies such as interferon alpha (INF-α) or interleukin 2 (IL-2) were associated with substantial toxicity and questionable effectiveness (5). The rationale for using agents such as INF-α in advanced RCC was based on evidence from two prospective randomized trials, SWOG-8949 (Southwest Oncology Group) and EORTC-3047 (by the European Organization for Research and Treatment of Cancer). Both showed a significant survival advantage and delayed time to disease progression in patients who underwent CN followed by immunotherapy versus patients undergoing immunotherapy alone with INF-α (6,7). The SWOG study included 241 patients and showed a 3-month survival benefit in the nephrectomy group versus non-nephrectomy group (11.1 vs. 8.1 months, respectively). The difference in median survival between the two groups was independent of performance status, metastatic site, and the presence or absence of a measurable metastatic lesion (6).
Likewise the EORTC study showed an even more pronounced benefit in patients undergoing CN followed by INF-α (study group) vs. INF-α alone (control group). All patients had mRCC that had been histologically confirmed and was progressive at entry. Fifty-three percent of patients received at least 16 weeks of INF-α treatment, which was also the median duration of treatment. Time to progression (5 vs. 3 months), and median duration of survival (17 vs. 7 months) were significantly better in study patients than in controls, respectively. Toxicity resulting in dose modification was necessary in 32% of patients, most commonly because of non-haematological side-effects (7). However, both studies showed very low perioperative mortalities of less than 1%.
A combined analysis of the above SWOG and the EORTC trials by Flanigan et al. showed an overall survival of 13.6 months for nephrectomy plus INF-α vs. 7.8 months for INF-α alone (8). This 6-month survival advantage represented a 31% reduction in risk death in the CN group. A subsequent update of the SWOG data with 9 years of follow-up, continued to favor CN showing a 3-month survival benefit in the nephrectomy group or a 26% reduction in death (9). Multivariate analysis showed that performance status 1 vs. 0, high alkaline phosphatase and lung metastasis only were overall survival predictors. This analysis also highlighted that patients who progressed within 3 months after CN did not appear to benefit from surgery. Thus, CN prolongs overall survival, supporting its role as standard therapy in patients with advanced RCC in the immunotherapy era.
CN with post-surgical targeted therapy
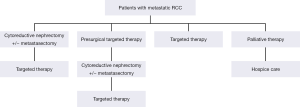
The introduction of various tyrosine kinase inhibitors (TKI’s) and other agents that target the VEGF and mTOR pathways have quickly replaced cytokines as the dominant systemic therapy in metastatic RCC. Several treatment strategies are now available for patients with metastatic RCC depending on both their performance and disease status (Figure 1). The benefits of targeted therapy over cytokine therapy include ease of administration, toxicity profile and superior efficacy in progression-free and overall survival (10). For example, targeted therapies used in patients who had not undergone CN still showed an improved treatment effect to standard immunotherapy. A randomized study of 626 patients by Hudes et al., showed that patients who received temsirolimus alone had longer overall survival and progression-free survival than patients who received interferon alone (11).
Despite these advantages, the majority of evidence supporting the integration of surgery and systemic therapy from the cytokine era with newer targeted therapy has yet to be established. Furthermore, such advances in the treatment of metastatic RCC have led some investigators to question the benefit of CN. A study by Tsao et al. utilizing the SEER (Surveillance, Epidemiology and End Results)—Medicare dataset from 2001-2008 showed a decreasing trend in the utilization of CN in the targeted therapy era suggesting a potential uncertainty in survival benefit of CN with newer available targeted agents (12).
A recent Cochrane review highlights over 13 trials out of 28 that showed improved progression free survival with new targeted agents. Over all, nephrectomy status did not appear to be essential to benefit from targeted therapy, however it is important to note that patients who did not undergo nephrectomy were likely to have important different characteristics and comorbid status compared to the surgical group (5,13). Other trials have also questioned the benefit of CN in the context of adjuvant targeted systemic treatment. You et al. reported that CN provided no survival benefit in 78 patients with mRCC in receiving TKI therapy with or without nephrectomy despite the median OS in the CN group was twice that compared to the in the non-CN group (21.6 vs. 13.9 months) (14). Another retrospective review by Richey et al., showed overall mean survival of 10.4 months in 188 patients with targeted therapy alone implying CN does not improve survival (15).
Even though these newer targeted treatments have revolutionized modern medical treatment of mRCC, these agents are not curative and complete responses are rare. In modern practice, despite the lack of level 1 evidence establishing the role of CN in patients receiving targeted therapies, CN continues to be an integral component of mRCC management. It is important to emphasize that the clinical trials that led to the approval of the seven current targeted agents available by the Food and Drug Administration (FDA), almost all patients had undergone prior nephrectomy, hence the benefits of such agents must be recognized within the context of a resected primary tumor (Table 1).
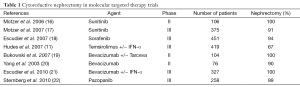
Full table
In contrast, a multi-institutional study by Choueiri et al. revealed a significant overall survival advantage in subjects undergoing CN with favorable and intermediate prognostic features described by Heng et al. (23) for targeted agents. These included performance score (PS) >80, age less than 75 years, more than one site of metastatic disease and absence of brain metastases (24). Only a marginal benefit was observed in those patients with poor risk features, reinforcing the need for risk stratification and prognosticators to identify patients who will benefit from CN. Similarly, a further study by Shuch et al. reinforces the relationship between PS and improved survival after CN (25). In this study, the median disease-specific survival for patients post-CN with ECOG PS of 0, 1, and 2/3 was 27, 13.8, and 6.6 months, respectively suggesting that surgery in patients who have a poor performance may serve a palliative function, but should be performed with caution due to poor outcomes within this group.
The optimal answer to whether CN will be of benefit in the era of targeted therapy may be fulfilled by the ongoing CARMENA phase III trial. The trial hopes to recruit 700 patients with the primary tumor in place, randomizing patients to nephrectomy followed by sunitinib or sunitinib alone. Ongoing accrual difficulties and the fact that since its inception, there are an increasing number of available targeted agents will make it difficult to generalize its findings to newer agents. Regardless, evidence from CARMENA may help bridge the gap between the immuno- and targeted therapy eras providing level 1 evidence that CN continues to be beneficial to patients with mRCC in combination with these newer agents.
Patient selection/risk stratification for CN in metastatic RCC
Despite evidence that CN prolongs survival in patients with metastatic RCC prior to INF-α or targeted therapy, there are certain subgroups of patients that do not benefit from surgery. Prognostic variables that allow clinicians to discern if patients that will benefit from therapy are paramount in risk stratification and patient counseling prior to commencing medical and or surgical therapy.
Many publications have examined postsurgical outcome in the pre and post-targeted therapy era (2,24,26-29). One of the most widely used models in mRCC is the Memorial Sloan Kettering Cancer Center (MSKCC) model derived from 400 patients treated with INF-α. This model utilizes LDH, corrected calcium, serum hemoglobin, Karnovsky patient performance status and time from diagnosis to start of therapy to risk stratify patients for survival (30,31). However, the MSKCC risk factors were created during the immunotherapy era and it is uncertain whether these are still useful in the era of targeted therapy.
In 2009, Heng et al. retrospectively reviewed 645 patients with metastatic RCC treated with targeted therapy. They identified six predictors of survival similar to the MSKCC criteria including hemoglobin below lower limit of normal (LLN), calcium above upper limit of normal (ULN), Karnofsky score ≤80% and systemic disease within 1-year of diagnosis as independent predictors of decreased survival. Absolute neutrophil count greater than ULN and platelets greater than ULN were also independent adverse prognostic factors. Based on these six prognostic factors, patients were risk stratified to favorable (0 adverse factors: 75% 2-year survival), intermediate (1-2 adverse factors: 53% 2-year survival), or poor (3-6 adverse factors: 7% survival) (23). Both Heng and MSKCC models are useful in extrapolating which patients may most benefit from CN.
Clearly, appropriate patient selection is critical to the successful integration of surgery with systemic therapy. Within the literature there are also many published nomograms to facilitate better patient selection to identify those unlikely to benefit from CN (32-35). Such models may be helpful in selecting patients for CN, but all are inherently limited in their clinical use due to their retrospective nature. In a large retrospective analysis, Culp et al. compared 566 patients who underwent CN and 110 patients who received medical therapy alone (33). Surgical patients who died within 8.5 months of CN did not appear to benefit from surgery versus medical therapy alone. Within this group the authors identified seven significant pre-operative variables that were negative predictors of survival. These included serum albumin below the LLN, serum lactate dehydrogenase level above the ULN, a clinical tumor classification of T3 or T4, symptoms at presentation caused by metastatic disease, the presence of liver metastasis and radiological evidence (≥1 cm) of retroperitoneal or supra-diaphragmatic adenopathy at time of CN. Patients who had ≥4 risk factors did not benefit from CN versus medical therapy alone (33).
Even though this retrospective study did not standardize patients to any specific targeted regime, these pre-operative risk factors may be a useful aid in identify patients for CN. Another study by Margulis et al. developed a multivariable model examining cancer specific survival in patients following CN in 601 patients identifying both pre-operative and post-operative variables using previously identified negative risk factors for survival including LDH, albumin, pathological tumor and nodal stage (32). Other factors that likely impact outcome in CN and targeted therapy include presence of sarcomatoid differentiation and non-clear cell histology within the nephrectomy specimen, which have both been associated with worse survival (33,36-38).
Lastly percentage of tumor volume removed, defined as ‘fractional percentage of tumor volume removed’ (FPTV) may also impact outcome of CN. Fallick et al. showed that within the immunotherapy era reduction of >75% of overall tumor burden was required to be beneficial (39). More recently, studies suggest a much higher threshold (>90%) of tumor debulking is required to improve progression free survival and overall survival. Barbastefano and colleagues reported that FPTV remained an independent predictor of progression free survival in patients treated with a combination of targeted molecular therapy (TMT)’s where the median FPTV removed was 95% (40).
Timing of CN
The timing of CN, though controversial, is still most commonly performed prior to the commencement of systemic therapy. With higher response rates of targeted agents especially within the metastatic setting, there is an increasing interest in assessing neoadjuvant use of these agents in RCC supporting the treatment paradigm of initial systemic therapy followed by consolidative surgery.
The argument for initiating targeted therapy prior to CN includes timely delivery of systemic therapy to the patients with metastatic disease, and potentially down staging of the primary tumor to facilitate future surgical extirpation. Furthermore, excision of the primary tumor may remove a source of immunosuppressive cytokines or growth factors that stimulate the progression of metastatic sites (41). Another advantage of pre-surgical targeted therapy is that it may act as a litmus test allowing for better patient selection. Patients that respond to systemic therapy are most likely to benefit from CN where as those that rapidly progress could avoid potential surgical morbidity.
For example, a phase II study from the immunotherapy era by Bex et al. (42) evaluated the response to immune therapy as a selection tool for subsequent CN in 16 patients newly diagnosed with metastatic RCC. Patients were treated with two cycles of low dose IL-2 and IFN-α prior to CN. Five patients (31%) had rapidly progressive disease and spared the morbidity of radical nephrectomy (RN) with a median survival of 3 months whereas 11 patients (69%) had tumor response or stability and underwent CN with a median survival of 11.5 months. In a follow-up study by Bex et al., IFN-α was administered to intermediate risk patients with metastatic RCC (43). Similar to the previous study, patients who had tumor response or stability after receiving IFN-α underwent CN whereas 50% patients rapidly progressed and were spared surgery. Such approaches clearly highlight the feasibility of pre-surgical systemic therapy as a litmus test for patient selection.
Recently several groups have reported successful use of targeted agents in patients with the primary tumor in situ (38,44-46). For example, a study by Thomas et al., daily sunitinib in 19 patients with locally advanced disease or metastatic RCC showed that after two cycles of therapy 16% of primary tumors demonstrated a partial response with an average shrinkage of 24%. Seven percent of patients had stable disease and 47% of tumors demonstrated progression (46). This same study highlighted four patients with locally advanced RCC were initially deemed unresectable due to the proximity to adjacent structures prior to medical therapy. After treatment with presurgical sunitinib, 3 out of the 4 patients were able to undergo nephrectomy with tumor shrinkage ranging between 11-24% (46).
In one of the largest retrospective series, Abel et al. reported 168 patients with metastatic RCC receiving targeted therapy with the primary tumor in situ resulting in a median tumor diameter shrinkage of 6.5 cm (7.1%) at 62 days. Most patients had a partial response or stable disease (59%) whereas 41% demonstrated disease progression (47). Other retrospective series also report similar tumor volume shrinkage between 24-31% with neoadjuvant targeted therapy such as sunitinib or sorafenib (45,48). Regardless, neoadjuvant therapy with any of the targeted agents have yet to be curative and the question arises whether the modest reductions in primary tumor burden is clinically meaningful. Furthermore, the definition of surgical resectability is poorly defined with subjective variability depending on surgeon, patient’s clinico-pathological and radiological parameters. In the modern era, less than 1% of cases are deemed unresectable (49).
The safety of pre-surgical targeted therapy in patients is also important. A study by Chapin et al. compared complications between 70 patients receiving neoadjuvant targeted therapy prior to CN versus patients who had immediate CN. The use of pre-surgical therapy in patients with metastatic RCC did not result in increased overall complication rates or complications requiring intervention (Clavien >3) when compared to patients undergoing immediate CN. However, an increased risk of wound complications was noted. Patients were also more likely to have late complications or multiple events especially wound related events in the neoadjuvant therapy setting (50).
The disadvantage of performing CN first is that disease progression may occur during recovery after surgery and the window of benefit from systemic therapy is missed. Both the SWOG and EORTC trial in the cytokine era reported that 20-25% patients rapidly progressed and died within 4/12 after CN suggesting overtreatment (6,7). Despite these concerns, CN will continue to remain the standard of care for many patients with metastatic RCC until integrating CN and targeted therapy is shown to be inferior to targeted therapy alone (51). The value of pre-surgical targeted therapy may be further clarified from the ongoing SURTIME trial (randomized phase 3 trial) where 458 patients will be randomly assigned to either immediate CN followed by sunitinib or three cycles of pre-surgical sunitinib followed by deferred CN.
Presurgical targeted therapy downstaging of inferior vena cava (IVC) thrombus
The timing of targeted therapy in patients with IVC involvement of locally advanced RCC prior to surgery must also be reviewed. Venous tumor thrombi are present in approximately 10% of patients with RCC (52) and surgery for such thrombi is associated with increased morbidity and mortality (53). With the cytoreductive effects of TMT for RCC, there is hope that such therapy could also decrease the tumor thrombus burden, in turn potentially reduce the extent of morbidity and mortality of surgery. The use of targeted therapy in RCC to downsize caval thrombus has been documented in various case reports (38,54,55) and even though such cases are memorable, the current literature is extremely limited.
A study by Cost et al. examined the role of pre-surgical targeted therapy in patients with IVC thrombus in 25 patients (56). Before targeted therapy, thrombus level was II in 18 (72%) patients, III in 5 (20%) patients, and IV in 2 (8%) patients. Following targeted therapy, 7 (28%) patients had a measurable increase in thrombus height, 7 (28%) had no change, and 11 (44%) had a decrease. One patient (4%) had an increase in thrombus-level classification, 21 (84%) had stable thrombi, and in 3 (12%) the thrombus level decreased. There was only 1 case (4%) where the surgical approach was potentially affected by tumor thrombus regression (level IV to III). No statistically significant predictors of tumor thrombus response to targeted therapy were found (56). This study implies that targeted therapy has minimal clinical effect on RCC tumor thrombi and CN and IVC thrombectomy should not be delayed in good surgical candidates.
Although the previous study is the largest reported experience within in situ caval tumor thrombi treated with TMT, most cases were treated with targeted therapy for reasons other than downsizing of the caval tumour thrombus and many of the patients were not even candidates for surgery. Furthermore, the current series lacks sufficient statistical power to adequately assess the usefulness of TMTs in tumor thrombus cytoreduction and further investigation is required (56).
Another retrospective study by Kwon et al., reviewed 45 patients with synchronous mRCC with IVC thrombus. Twenty-eight patients underwent RN with IVC thrombectomy followed by targeted therapy and 17 received targeted therapy alone. Progression-free and overall survival were similar in both groups and surgical resection of the primary renal mass with IVC thrombus did not appear to affect the probability of progression or overall mortality suggesting a limited role for surgery in this patient population (57). In summary, the survival advantage of targeted therapy in the adjuvant setting after nephrectomy and IVC thrombectomy still remains to be further investigated with little in the literature to guide clinicians.
Cytoreductive surgery with metastasectomy
In selected patients with low volume metastatic RCC, surgical resection of metastatic foci can yield long-term disease-free survival, where metastasectomy may be performed concurrently with RN or shortly after. A study by Eggener and colleagues reported clinical benefit of metastasectomy in 44 patients across all three MSKCC risk categories in both the synchronous and metachronous metastatic settings. On multivariate analysis a better risk category and metastasectomy were each independently associated with more favorable survival (58).
Alt et al. described outcomes of complete metastasectomy in 125 patients (59). This study showed that complete metastasectomy was associated with a significant prolongation of median CSS (4.8 vs. 1.3 years). Patients who had lung-only metastases had a 5-year CSS rate of 73.6% with complete resection versus 19% without complete resection On multivariate analysis, the absence of complete metastasectomy was associated significantly with an increased risk of death from RCC (hazard ratio 2.91) (59). The authors conclude that complete resection of multiple RCC metastases may be associated with long-term survival and should be considered when technically feasible in appropriate surgical candidates.
Another study by Russo et al., described 61 patients undergoing CN with complete metastasectomy during the immunotherapy era in patients with involvement of single and multiple organ sites (60). Median survival was 30 months in patients who underwent CN and complete metastasectomy compared to patients who underwent CN alone (median 12 months). More recently, Karam et al. reported on 22 patients who underwent consolidative metastasectomy after at least one cycle of targeted therapy. Fifty percent of patients remained disease free at a median of 10 months. Even though these patients were highly selected with limited disease burden, this study contributes further evidence of the feasibility of consolidative metastasectomy with acceptable morbidity in the TMT era (61). To date, even though evidence favors a survival advantage for metastasectomy in the TMT era in selected patients, the true benefit of adjuvant targeted therapy after metastasectomy still warrants further investigation.
Lastly, a recent sub-sectional analysis from the only systematic review within the literature, identified eight studies that assessed metastases from various organs examining complete metastasectomy versus no metastasectomy or both. The majority reported a significantly longer CSS and OS with complete metastasectomy compared with no metastasectomy or incomplete metastasectomy (median of medians 40.8 vs. 14.8 months, respectively). A summary of survival outcome using forest plot hazard ratios for CSS and OS regardless of organ site, unequivocally favored complete metastasectomy in all eight studies (62).
Conclusions
In conclusion, cytoreductive surgery continues to play an important role in the era of TMT. The largest survival benefit of CN in mRCC is seen in patients with favorable risk categories according to the MSKCC/Heng criteria and especially in patients where a high percentage of tumor burden can be removed. Patient selection is paramount in the decision to perform CN judiciously, as some patient will not benefit due to rapidly progressive disease. Surgery should be based upon volume of resectable disease, performance status, and other prognostic features. Prognostic models developed based on patients treated with targeted agents may enhance our ability to select patients who will gain the most benefit from surgical debulking. It is likely that a subset of patients with poor risk disease treated with upfront systemic therapy will benefit from delayed CN. Currently ongoing clinical trials should help to further define the role of CN in the era of TMT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J 2008;14:288-301. [PubMed]
- Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004;22:454-63. [PubMed]
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353:2477-90. [PubMed]
- Patard JJ, Pignot G, Escudier B, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol 2011;60:684-90. [PubMed]
- Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011;108:1556-63. [PubMed]
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001;345:1655-9. [PubMed]
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358:966-70. [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [PubMed]
- Lara PN Jr, Tangen CM, Conlon SJ, et al. Predictors of survival of advanced renal cell carcinoma: long-term results from Southwest Oncology Group Trial S8949. J Urol 2009;181:512-6; discussion 516-7. [PubMed]
- Crispen PL, Blute ML. Role of cytoreductive nephrectomy in the era of targeted therapy for renal cell carcinoma. Curr Urol Rep 2012;13:38-46. [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [PubMed]
- Tsao CK, Small AC, Kates M, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy in the United States: a SEER analysis. World J Urol 2013;31:1535-9. [PubMed]
- Coppin C, Le L, Porzsolt F, et al. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev 2008.CD006017. [PubMed]
- You D, Jeong IG, Ahn JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol 2011;185:54-9. [PubMed]
- Richey SL, Culp SH, Jonasch E, et al. Outcome of patients with metastatic renal cell carcinoma treated with targeted therapy without cytoreductive nephrectomy. Ann Oncol 2011;22:1048-53. [PubMed]
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516-24. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. Erratum in: N Engl J Med 2007;357:203. [PubMed]
- Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol 2007;25:4536-41. [PubMed]
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427-34. [PubMed]
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [PubMed]
- Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60-6. [PubMed]
- Shuch B, La Rochelle JC, Wu J, et al. Performance status and cytoreductive nephrectomy: redefining management in patients with poor performance. Cancer 2008;113:1324-31. [PubMed]
- Choueiri TK, Rini B, Garcia JA, et al. Prognostic factors associated with long-term survival in previously untreated metastatic renal cell carcinoma. Ann Oncol 2007;18:249-55. [PubMed]
- Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol 2005;174:1759-63; discussion 1763.
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;97:1663-71. [PubMed]
- Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;98:2566-75. [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40. [PubMed]
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289-96. [PubMed]
- Margulis V, Shariat SF, Rapoport Y, et al. Development of accurate models for individualized prediction of survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Eur Urol 2013;63:947-52. [PubMed]
- Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer 2010;116:3378-88. [PubMed]
- Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 2008;113:1552-8. [PubMed]
- Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 2011;60:644-61. [PubMed]
- Kassouf W, Sanchez-Ortiz R, Tamboli P, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma with nonclear cell histology. J Urol 2007;178:1896-900. [PubMed]
- Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013;119:2999-3006. [PubMed]
- Shuch B, Riggs SB, LaRochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int 2008;102:692-6. [PubMed]
- Fallick ML, McDermott DF, LaRock D, et al. Nephrectomy before interleukin-2 therapy for patients with metastatic renal cell carcinoma. J Urol 1997;158:1691-5. [PubMed]
- Barbastefano J, Garcia JA, Elson P, et al. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int 2010;106:1266-9. [PubMed]
- Thomas AA, Rini BI, Campbell SC. Integration of surgery and systemic therapy in the management of metastatic renal cancer. Curr Urol Rep 2009;10:35-41. [PubMed]
- Bex A, Horenblas S, Meinhardt W, et al. The role of initial immunotherapy as selection for nephrectomy in patients with metastatic renal cell carcinoma and the primary tumor in situ. Eur Urol 2002;42:570-4; discussion 575-6. [PubMed]
- Bex A, Kerst M, Mallo H, et al. Interferon alpha 2b as medical selection for nephrectomy in patients with synchronous metastatic renal cell carcinoma: a consecutive study. Eur Urol 2006;49:76-81. [PubMed]
- Amin C, Wallen E, Pruthi RS, et al. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology 2008;72:864-8. [PubMed]
- Jonasch E, Wood CG, Matin SF, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:4076-81. [PubMed]
- Thomas AA, Rini BI, Lane BR, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol 2009;181:518-23; discussion 523. [PubMed]
- Abel EJ, Culp SH, Tannir NM, et al. Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol 2011;59:10-5. [PubMed]
- van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res 2008;14:2431-6. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [PubMed]
- Chapin BF, Delacroix SE Jr, Culp SH, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol 2011;60:964-71. [PubMed]
- Thomas AA, Campbell SC. Consolidative surgery after targeted therapy for renal cell carcinoma. Urol Oncol 2013;31:914-9. [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Karnes RJ, Blute ML. Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol 2008;5:329-39. [PubMed]
- Bex A, Van der Veldt AA, Blank C, et al. Progression of a caval vein thrombus in two patients with primary renal cell carcinoma on pretreatment with sunitinib. Acta Oncol 2010;49:520-3. [PubMed]
- Peters I, Winkler M, Jüttner B, et al. Neoadjuvant targeted therapy in a primary metastasized renal cell cancer patient leads to down-staging of inferior vena cava thrombus (IVC) enabling a cardiopulmonary bypass-free tumor nephrectomy: a case report. World J Urol 2014;32:245-8. [PubMed]
- Cost NG, Delacroix SE Jr, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 2011;59:912-8. [PubMed]
- Kwon T, Lee JL, You D, et al. Impact of surgery on the prognosis of metastatic renal cell carcinoma with IVC thrombus received TKI therapy. J Surg Oncol 2014;110:145-50. [PubMed]
- Eggener SE, Yossepowitch O, Kundu S, et al. Risk score and metastasectomy independently impact prognosis of patients with recurrent renal cell carcinoma. J Urol 2008;180:873-8; discussion 878. [PubMed]
- Alt AL, Boorjian SA, Lohse CM, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011;117:2873-82. [PubMed]
- Russo P, Synder M, Vickers A, et al. Cytoreductive nephrectomy and nephrectomy/complete metastasectomy for metastatic renal cancer. ScientificWorldJournal 2007;7:768-78. [PubMed]
- Karam JA, Rini BI, Varella L, et al. Metastasectomy after targeted therapy in patients with advanced renal cell carcinoma. J Urol 2011;185:439-44. [PubMed]
- Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 2014;15:e549-61. [PubMed]