Mechanisms of resistance in castration-resistant prostate cancer (CRPC)
Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy in men and remains the second-leading cause of cancer-related death in men (1,2). Despite advances in screening for and early detection of prostate cancer, a large portion of men continue to present with advanced or metastatic disease-approximately 20% of men in recent reports (3). Indeed, the morbidity from this disease remains high, with more than 29,000 prostate cancer related deaths in 2013 alone (1).
Androgen deprivation therapy (ADT), the standard of care for patients with biochemical recurrence after definitive primary therapy, locally advanced disease or metastatic disease, has been demonstrated to provide an initial benefit, but the majority of patients will progress to castration-resistant disease within 2-3 years (4).
Castration-resistant prostate cancer (CRPC), previously called hormone-refractory prostate cancer, is now understood to be a progression of disease despite medical or surgical castration. The paradigm shift is due to the understanding that CRPC is not hormone-refractory—in fact, the androgen axis continues to play an important role in the function and growth of CRPC. Indeed, while other pathways can contribute to castration-resistance, the androgen receptor (AR) remains the most important driver in the continuum of CRPC.
Understanding the mechanisms of resistance that cause hormone-naive prostate cancer to progress to castration-resistance is the key to developing future therapy. In this review, we will review the current knowledge regarding the mechanisms leading to castration resistance, the agents currently available for treatment of CRPC, and the mechanisms of resistance against these agents.
Background
Understanding the androgen axis is a key component to understanding the mechanisms by which castration-resistance develops.
Androgen receptor (AR)
The AR gene on Xq11-12 encodes for a 110 kDa nuclear receptor with four distinct functional motifs—the amino-terminal domain (NTD), DNA-binding domain, hinge region, and ligand-binding domain (LBD) (5-7). The cytoplasmic receptor is bound by heat-shock proteins (specifically HSP90 chaperone complex) in the inactive state (8). Androgen binding, specifically dihydrotestosterone (DHT) or testosterone, to the LBD causes a conformational change that leads to dissociation of the HSP90 complex, homo-dimerization of the receptor, translocation to the nucleus, and binding to androgen-response elements (AREs) in the promoter region of androgen-regulated genes (6,9). This interaction with the promoter region is under the influence of many transcriptional coregulators. Over 150 proteins have been identified (10), and many are enzymes (histone acetyltransferases, methyltransferases, kinases) that act to open the chromatin structure to promote transcription.
Androgens
Prostate cancer growth and survival depends on androgens, the major ligands for the AR. Testosterone is the primary circulating androgen, with approximately 90% produced by Leydig cells in the testes and 10% produced by the adrenal cortex. Only a small portion (3%) of circulating testosterone is unbound and functionally active—the remainder is bound and sequestered by sex-hormone binding globulin and albumin. However, testosterone is not the primary functionally active androgen in the prostate microenvironment. Following diffusion into the cytoplasm, testosterone is converted by the enzyme 5α-reductase to DHT, which has a five-fold higher affinity for the LBD of AR (11-13).
Physiologic levels of androgens are required to promote growth and prevent apoptotic death. Therefore, the pathways under AR influence are varied, but focus on the functions of the luminal epithelial cells, including production of seminal fluid proteins such as prostate-specific antigen (PSA) and multiple genes in the metabolic pathway leading to increased protein and lipid synthesis (14-16).
Steroidogenesis, which leads to androgen production, is an important pathway to understand, as it can be fundamentally altered in CRPC. Testosterone is produced by the testes and adrenal gland, and then converted in the cytoplasm to DHT via the activity of 5α-reductase (17). However, in the presence of ADT, studies have demonstrated persistent levels of intratumoral DHT (18-21), suggesting that altered steroidogenesis pathways have been activated (20). Adrenal testosterone sources, unaffected by ADT, and intratumoral de novo androgen synthesis may be sources of persistent ligand-dependent AR activity in CRPC (22).
Androgen deprivation therapy (ADT)
Since Huggins and Hodges (23) first demonstrated the dependence of prostate cancer on androgen signaling, ADT through either medical or surgical castration has been the standard of care for metastatic and locally advanced disease. Surgical castration, or bilateral orchiectomy, removes testicular androgens from circulation by removal of the source. Medical castration is achieved through the use of different classes of agents. LHRH agonists and antagonists deplete the pituitary production of luteinizing hormone (LH) through negative feedback or competitive inhibition, respectively, which in turn prevents testicular testosterone production (24). Anti-androgens work as competitive inhibitors at the LBD of AR, thereby preventing androgen stimulation of AR. These agents, in conjunction, provide complete androgen blockade (25,26).
Castration resistance
Despite the initial response to androgen blockade, all patients will eventually progress to castration resistance. Castration resistance is progression of disease, either clinical (development of metastatic disease, progression of pre-existing disease) or biochemical (three consecutive rises in PSA levels above nadir) in the presence of castrate levels of circulating testosterone (<50 ng/dL) (27,28).
Indeed, the biochemical recurrence of PSA, an AR regulated gene measured by serum levels, is evidence that CRPC is not hormone-insensitive. When adding first generation anti-androgens, such as flutamide or bicalutamide, to the treatment regimen of patients with advanced or metastatic disease, a decrease in serum PSA is often initially noted, indicating a response to direct AR blockage (29,30). However, serum PSA levels will again rise despite anti-androgen therapy, suggesting that the agent has begun functioning as an AR agonist; this is validated by the PSA decrease noted with anti-androgen withdrawal (31,32).
Further evidence for the critical role of the androgen axis in the development of CRPC lies in the finding that, despite castrate levels of serum testosterone, there remains a higher level of intra-tumoral androgens in CRPC compared to hormone-naïve prostate cancer (18-21). Recent studies have demonstrated that intra-tumoral androgen levels in CRPC are similar to those of eugonadal men, and in some cases even increased (22,33).
The AR persists in CRPC cells, and the re-activation of this axis by the following mechanisms appears to drive progression to castration-resistance.
AR dependent mechanisms of resistance leading to CRPC
The majority of mechanisms identified leading to castration-resistant are mediated by AR or the androgen axis. As seen in Figure 1, they can be categorized into five main subsets.
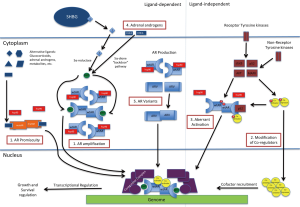
AR amplification and mutations/hypersensitivity pathway/promiscuous pathway
Low levels of androgen persist despite androgen blockade with ADT. Within this microenvironment, a subset of cells develop sensitivity to these low levels of androgens either through amplification of the AR (hypersensitivity pathway) (34) or development of AR mutations that lead to activation by molecules other than androgens (promiscuous pathway) (35,36).
Amplification of the AR has been identified in a significant portion of CRPC cell lines, ranging from 30-80% (37,38). This finding is uncommon in hormone naïve prostate cancer and may be due to selective outgrowth of CRPC cells (36). This amplification enables CRPC to be hypersensitive to low level of androgens, which promotes progression of disease (35). As 20% of CRPC metastases have evidence of AR amplification, which is absent in hormone-naive metastatic disease, it may also contribute to metastases. In addition, recent studies have shown that exogenous overexpression of AR can lead to CRPC.
Related to this concept, a substitution of valine with leucine at codon 89 results in increased 5α-reductase levels in a subset of CRPC. This results in higher levels of DHT despite low circulating levels of testosterone. This mutation is more commonly observed in the African-American population, and has been associated with more aggressive, early onset prostate cancer (39,40).
There have been various point mutations identified in the AR gene itself that lead to increased AR activity in the presence of low levels of androgens as well as non-androgenic steroids, such as progesterone, hydrocortisone, estradiol, and certain AR antagonists. The substitution of threonine with alanine at codon 877 in LNCAP cells (41,42) and the substitution of histidine for tyrosine in CWR22 cells (43,44) are well described in the literature; other examples include L701H, V715M, W741C (45-47). While most of the mutations are predominantly in the LBD, mutations in the NTD and DNA-binding domain were also identified (48,49).
Co-activators and co-repressors
Over 150 different molecules have already been identified as co-activators and co-repressors for AR (10). The AR normally recruits a series of coregulator complexes, which can function to either enhance (co-activators) or repress (co-repressors) transcriptional activity. Many of these coregulators are enzymes that serve to modulate other proteins in the complex, either through phosphorylation, methylation, acetylation or ubiquitylation, but they have also been identified as molecular chaperones, recruiters of transcriptional machinery and RNA splicing regulators (50-52).
One coactivator, FKBP51, which is also an AR target gene, was found to be upregulated in relapsed LAPC-4 tumors grown in castrate mice (53). It promotes formation of a superchaperone complex by regulating the recruitment of p23, a co-chaperone, to ATP-bound Hsp90, which in turns keeps AR in a conformation with high-affinity for ligand binding. This promotes androgen-stimulated transcriptional activity and growth.
The steroid receptor coactivators (SRC) are a class of AR coactivators capable of acetyltransferase activity, which in turn enhances AR-induced transcription by promoting formation of complexes between AR-associated enhancers/promoters and the transcription start site of AR target genes (54). The SRC class includes SRC-1, SRC-2 (TIF-2, GRIP-1, NcoA2), and SRC-3 (AIB). Xu et al. demonstrated that all three have been associated with prostate cancer progression (55). Ueda et al. identified SRC-1, when phosphorylated by MAPK under the influence of IL-6, was capable of both ligand-dependent and ligand-independent AR activation (56). SRC-3, in particular, tends to be over amplified in human cancers. Chung et al. demonstrated that SRC-3 is not overexpressed in androgen-dependent prostate cancer, but is overexpressed in poorly differentiated and more advanced prostate cancer, and is directly associated with prostate cancer progression—SRC-3 knockout mice were effectively arrested at the well-differentiated stage and unable to progress to poorer pathology (57).
Other important pathways include p300/CBP, which promotes androgen-independent IL-6 mediated AR activation in the presence of STAT3 (58), and LSD1 and JMJD2c, lysine demethylases that demethylate the histone H3 proteins and lead to increased AR induced transcription (59). Many of these molecules have demonstrated AR-dependent and AR-independent effects, since their interaction is not limited to AR. Co-repressor proteins, on the other hand, have been found at reduced levels in CRPC.
Aberrant activation (post-translational modification)/outlaw pathway
While all the prior mechanisms mediate increased AR activity in the presence of ligand, ligand-independent AR activation is also an important mechanism of progression to castration-resistance. Various in vitro studies have suggested that multiple growth factors, cytokines, and kinase pathways increase AR signaling, thereby promoting progression to castration resistance in a ligand-independent manner (60). Identification and characterization of those ligand-independent pathways can lead to additional targeted therapies.
The NF-κB family of proteins has been established as an important component of the oncogenic pathway in multiple human malignancies. There are five distinct NF-κB proteins, including the well-studied p65/p50 heterodimer, which has been shown to be constitutively active in prostate cancer. Another of the NF-κB pathways, the p100/p52 pathway has been of recent interest. The processing of p100 to p52 via molecules such as lymphotoxin β, B-cell activating factor, CD40 ligand, and stat3 (61) in prostate cancer, leads to significant hyperplasia and induced castration-resistant growth. This was accomplished by limiting ADT mediated apoptosis and cell cycle inhibition, but was done so in the presence of continued AR expression and activation, which suggested that p52 may activate AR during the progression of CRPC. p52 mediated its effects in an AR dependent manner by interacting directly with the NTD of AR. Downregulation of p52 in C4-2 cells led to the loss of constitutive activation of AR which suggested an androgen independent activation of AR (62).
The PI3K pathway is another important player in this process. The loss of the tumor suppressor PTEN protein, which is a negative inhibitor of the PI3K/AKT pathway, is identified in nearly all metastatic prostate cancers. Its activation has been associated with development of CPRC in various preclinical models (63-65). PI3K, specifically the p110β isoform, has been strongly associated with prostate cancer growth and progression, through basal activation of AKT in prostate cancer models. The PI3K/AKT pathway is downstream of key receptor tyrosine kinases (RTKs) such as EGFR, IGFR, c-met, but some studies suggest independent activation of this pathway (66). While it is also upstream of some critical signaling proteins, such as mTOR, it has also been found that AKT directly phosphorylates AR at two locations, Ser-217 and Ser-791, particularly in a castrate-state, though the clinical significance is not yet certain (67).
Src kinase, the key member in the family of non-RTK called Src family of kinases (SFK), has been a focus of our lab and our collaborators. Src, in the 25 years since its discovery as the first proto-oncogene identified, has been targeted in the treatment of multiple other malignancies (68,69). Our research into the role of Src in prostate cancer identified Src as a key molecule in multiple pathways that allow for progression of prostate cancer (70,71). Src is expressed in commonly used CaP cell lines CWR22Rv1, DU145, LAPC-4, LNCaP, and PC-3. As Src is not constitutively active, it has been difficult correlating Src protein expression levels with cell proliferation or aggressiveness in vitro. However, Src kinase is downstream of many important prostate-cancer influences—as it is activated by growth factors, cytokines, chemokines, and gastrin-releasing peptide, it has a pleiotropic effect on prostate cancer (68,70,71). Our laboratory group demonstrated that higher relative Src activation was associated with worse prostate cancer phenotypes, specifically DU145 and PC3, and the use of a novel SRC inhibitor AZD0530 helped elucidate a few of the pathways mediated by Src in prostate cancer cell lines (70). Activation of Src kinase has been linked to androgen-independent cell growth (72-74), inhibition of anti-apoptotic pathways (75-77), cell migration and adhesion (73), and tumor invasion (78), among other aspects of prostate cancer cell biology. Based on this preclinical data, AZD0530 (saracatinib) was taken to phase II clinical trial, but it was demonstrated to have minimal clinical efficacy as monotherapy (79). Lack of clinical efficacy was also noted with dasatinib; in the phase III clinical trial of docetaxel with dasatinib or placebo in chemotherapy-naïve CRPC patients, there was no improvement in overall survival (80). Other non-tyrosine kinases, such as Btk and Etk within the Tek-family of non-tyrosine kinases, are being targeted as well; recent work by Guo and colleagues demonstrated that CTN06, a novel dual inhibitor of Btk and Etk, induced apoptosis and autophagy, and also re-sensitized cell lines to docetaxel (81).
Growth factor pathways, such as IGF and KGF, bind and activate AR in a castrate state. Growth factor receptors, such as IGF-1R, IL-6R, and EGFR, control critical downstream growth and survival pathways such as MAPK, PI3K/AKT, and STAT signaling. Various RTKs, such as Her-2/neu, EGFR, and IGR-1R, enhance AR stability and activity, and in some cases, promote androgen independence. Her2/neu, for example, was found to promote xenograft cell growth via Ack1-kinase, which phosphorylates AR at tyrosine-267 and activates it (82). Targeting these pathways has shown some promise—cabozantinib (XL-184) inhibits tyrosine kinases of c-Met and VEGF, and in phase II clinical trial, demonstrated significant benefit specifically for CRPC patients with bone metastases; However, it did not reach its primary endpoint (bone pain alleviation) in phase III trial, with no significant difference in bone pain alleviation between the treatment and control (mitoxantrone/prednisone) arms.
Altered steroidogenesis
CRPC develops in the presence of castrate-levels of circulating androgens. However, intra-tumoral levels of androgens in CRPC models have been established to be the same as or even higher than in eugonadal men, suggesting that there is alternative androgen production (18-22,33). This is likely due to adrenal production, specifically of androgen precursors of adrenal origin such as dehydroepiandrosterone (DHEA) and its sulfated form (DHEA-S), which can be converted to the highly active DHT via a “backdoor” pathway (83,84).
DHEA and DHEA-S, produced by the adrenal gland, are not affected by ADT and are still found in circulation. The molecules are converted to androstenedione (AD) either in the prostate or adrenal gland by 3βHSD, encoded by HSD3B. There are 2 isoforms, 3βHSD1 in the prostate and peripheral tissues, 3βHSD2 in the adrenal gland (85). The subsequent conversion from AD to DHT, in the absence of ADT, typically goes through testosterone as an intermediary, and requires 17βHSD3 and AKR1C3 (encoded by HSD17B3 and AKR1C3 respectively) and steroid-5α-reductase (two isoforms, encoded by SRD5A1 and SRD5A2). However, in the presence of ADT, the sequence can be reversed, leading to 5α-AD (5α-dione) serving as the intermediary between AD and DHT, bypassing testosterone completely. This alternative pathway, referred to as the “5α-dione” pathway, has been demonstrated to predominate in CRPC (86,87).
In addition to utilization of weak adrenal androgens in the 5α-dione pathway, recent assessment of CRPC cells has identified increased expression of steroidogenic enzymes such HSD3B1, HSD3B2, HSD17B3, AKR1C3, and SRD5A1 (20,87-89), which may contribute to de novo production of steroids and androgens. Up-regulation of SRD5A1 and concurrent down-regulation of SRD5A2 leads to higher levels of 5α-reductase-1, for which AD is a better substrate than testosterone (90-92). What drives the changes in transcription of these steroidogenic enzymes? Many single-nucleotide polymorphisms have been identified within the above enzymes, especially HSD3B1 and HSD3B2 (93), but the clinical significance of these is not yet clear.
AR variants
A more recent development has been the identification of splice variants of the AR (AR-Vs), which are constitutively active, typically due to the loss of the C-terminal LBD (94-97). Indeed, the amplification of AR seen in CRPC may contribute to the development of the splice variants. Most CRPC cell lines demonstrate low levels of AR-V, but 22RV1 express levels similar to full-length AR (44).
The functional implication of these variants is not yet fully understood. Direct measurement of splice variants has been limited by the lack of variant-specific antibodies, leaving only secondary assessment via RNA levels. ARV7 is the only variant that has a suitable antibody for staining, and immunohistochemistry staining has established increased expression in CRPC (95,96). However, transcribed RNA levels may not be completely reflective of protein levels. This suggests some post-translational control that has not yet been fully elucidated.
However, Hörnberg et al. reported high levels of splice variant expression in bone metastases compared to hormone-naïve prostate cancer, and that it led to CRPC and poorer prognosis (98). This study also demonstrated a discrepancy between RNA levels and protein levels, contributing to the difficulty in determining splice variant significance in CRPC development and progression.
The predominant variants are ARV1, ARV7 and ARV 567. Of the variants, ARV7 has been studied most extensively (95,96). As described above, it lacks the LBD, is located in the nucleus, and is constitutively active. It has been show to regulate both AR-regulated genes and a unique set of AR-independent genes (96), suggesting it has an overlapping but distinct role compared to full-length AR in prostate cancer cells (94).
A recently discovered variant, ARV8, actually lacks a DNA-binding domain. Therefore, it remains in the plasma membrane and its constitutive activity is limited to activation of cell signaling pathways (99). For example, Yang et al. demonstrated increased AR phosphorylation via an EGF-mediated SRC activation in the presence of this variant; its subsequent knockdown was associated with loss of this phosphorylation.
Mechanisms of resistance to current CRPC treatments
Based on this understanding of the development of CRPC, there are now approved medications for the management of patients who are castration-resistant. However, despite these new agents, all patients will eventually progress in their disease. Understanding the means by which prostate cancer overcomes these treatment modalities will help identify new treatment options.
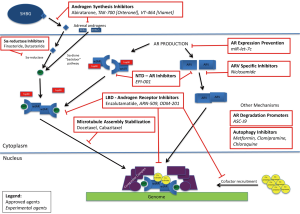
Below, we will address the primary agents currently available, focusing on their mechanism of action and current knowledge about the resistance to their function. Figure 2 provides an overview of the current and experimental agents affecting the androgen axis. As can be expected, there is crossover in many of these mechanisms, with these shared pathways being potentially significant future targets.
Docetaxel
Docetaxel is the current standard of care for patients who have progressed to castrate-resistant prostate cancer. SWOG 9916 and TAX327 demonstrated a 3-month survival advantage with docetaxel over mitoxantrone in CRPC patients (100-102), and until recently, it was the only approved primary therapy for CRPC. The recent CHAARTED trial, however, may have demonstrated a role for docetaxel as an initial treatment option for hormone-naïve prostate cancer in conjunction with ADT, as the combination was found to have a 17-month survival advantage (103).
Docetaxel is a well-known and studied chemotherapeutic agent used in the treatment of a variety of malignancies. It is an anti-mitotic chemotherapeutic agent that works by binding the β subunits of tubulin in microtubules, thereby stabilizing them and preventing the depolymerization required for mitosis (104-106), which induces apoptosis. In CRPC specifically, docetaxel leads to phosphorylation of bcl-2 (B-cell lymphoma 2), which causes caspase activation and apoptosis in vivo and in vitro (107,108). Additionally, AR expression is reduced in docetaxel-treated CRPC cells and is thought to be due to AR nuclear localization and inhibition of signaling (109).
Drug-efflux in CRPC enables resistance to docetaxel. Multi-drug resistance proteins (MDRP) are well described in the literature, and include P-glycoprotein (P-gp), multidrug resistance protein 1 (MRP1), and breast cancer resistance protein (BRCP). These molecules cause active efflux of multiple therapeutic agents. DU145 and 22RV1 cell lines, when made docetaxel-resistant, have been found to over-express P-gp (110), while CRPC lines exposed to docetaxel have been found to have MDR1 genetic variations that are more docetaxel-resistant (111). Docetaxel-resistant CRPC lines also upregulate the class III β-tubulin isoform, which allows less taxane binding. Inhibiting class III β-tubulin restores docetaxel sensitivity in those same cells (112,113). In addition, LNCAP derived docetaxel-resistant cells demonstrated an F270I mutation in the class I β-tubulin, which had stronger taxane binding at baseline (114).
While the above mechanisms are docetaxel-specific, other mechanisms of resistance have been identified. Docetaxel resistance has been linked to apoptosis pathways, specifically upregulation of p53 and activation of PAR1. p53 is an important cell cycle regulator, often found over-expressed in prostate cancer. LNCAP cells over-expressing wild type p53 are more resistant to docetaxel activity than DU145 and PC3 cell lines, which have reduced or no p53 activity (115). Zhu et al. demonstrated this in docetaxel-resistant C42B cells in vitro—cells treated with docetaxel had p53 phosphorylation and activation, but taxane-resistant C42B demonstrated no phosphorylation (116). PAR1, through NF-κB activation, has been shown to reduce docetaxel-induced apoptosis (117).
In addition to blocking docetaxel-induced apoptosis, docetaxel’s anti-mitotic activity itself directly initiates survival pathways in prostate cancer cell lines. Binding to the microtubules initiates pathways such as c-Jun N-terminal kinase (JNK), which in turns leads to activation of various transcription factors such as STAT-1, STAT-3, and NF-κB. Knockdown models of these transcription factors have been shown to be more sensitive to docetaxel-cytotoxicity (115,118).
Over-expression of cytokines and chemokines, such as IL-6, IL-8, and CCL-2, and chaperone molecules, such as HSP27 and HSP90, have been associated with docetaxel resistance, but no clinically significant inhibitors of these pathways have yet been identified. OGX-011, a second-generation antisense drug that inhibits the secretion of clusterin, a chaperone protein, was administered in conjunction with docetaxel in phase III trials, but did not meet its primary endpoint. Its activity focuses on CLU, a key protein that exists in two forms: nuclear CLU (nCLU) and secreted CLU (sCLU)—nCLU promotes docetaxel-mediated cell death while sCLU prevents it (119,120). Upon initiation of chemotherapy, especially docetaxel in prostate cancer cells, there is a shift in the balance towards sCLU, thought to be attributed to STAT-1 activation (110,121). However, inhibition of sCLU using antisense oligonucleotide re-sensitizes the cells to docetaxel (121,122).
Our lab group identified >1,600 genes that had altered expression in taxane-resistant C42B cells, with approximately 52% being upregulated. From this subset, we recently identified ABCB1, which belongs to the ATP-binding cassette (ABC) transporter family, among the top upregulated genes in the taxane-resistant cells. ABCB1 was highly expressed in taxane-resistant C42B cells, but virtually undetectable in taxane-sensitive C42B cells. Inhibition of ABCB1 expression resensitized C42B cells to docetaxel, and this was then confirmed in the DU-145 cell line (116). Apigenen, a natural molecule in the flavone family identified by Shukla and Gupta (123), was demonstrated to help resensitize cells to docetaxel therapy.
Abiraterone and androgen synthesis inhibitors
Abiraterone acetate (Zytiga) is a molecule structurally similar to pregnenolone that acts as an irreversible inhibitor of cytochrome p450, family 17, subfamily A, polypeptide 1 (CYP17A1). CYP17A1 is a member of the cytochrome p450 class of enzymes that serve as a catalyst for the oxidation of a variety of molecules. It has two consecutive enzymatic functions in the steroidogenesis pathway that contribute to the conversion of pregnenolone to DHT. Loss of CYP17A1 activity causes significant loss of androgen production in the peripheral organs, particularly adrenal androgens. It has been found to be 10-30 times more potent than ketoconazole, which is a non-specific inhibitor of p450 enzymes and previously has been used to generate rapid androgen ablation (106). The phase III trial COU-AA-301 demonstrated a 3.9-month survival benefit of abiraterone/prednisone over placebo/prednisone in patients who had progressed on docetaxel therapy (124). The subsequent COU-AA-302 trial demonstrated benefit in the pre-chemotherapy space, with improved radiographic progression free survival, time to initiation of chemotherapy, and a trend towards improved overall survival (125).
Altered steroidogenesis was discussed as one mechanism by which CRPC develops. While abiraterone-naive CRPC cell lines utilize the “5α-dione” pathway to generate intra-tumoral DHT by bypassing testosterone, they are still dependent on adrenal androgens. By irreversibly inhibiting this critical upstream enzyme in the steroidogenesis pathway, abiraterone effectively causes a significant decrease in intra-tumoral androgen levels by preventing production of adrenal androgens.
However, despite its effectiveness in inhibiting the steroidogenesis pathway (126), abiraterone’s effect is incomplete. Attard et al. demonstrated that while most urinary androgen metabolites and serum androgens were suppressed, the inhibition of CYP17 led to higher levels of urinary metabolite 3α5α-17HP, which correlated with the excretion of androsterone—which is the primary metabolite of 5α-reducted androgens such as DHT (127). This suggests that the use of abiraterone may push 17-hydroxyprogesterone towards the “5α-dione” pathway.
As can be expected, over-expression or mutations of CYP17A1 may also contribute to abiraterone resistance (128). Chang et al. demonstrated that the HSD3B1 (1245C) mutation previously mentioned as contributing to progression to CRPC has also been found in abiraterone-resistant xenograft models, though the clinical significance of this still needs to be elucidated (93). Mostaghel et al. demonstrated that abiraterone-treated cell lines responded with increased expression of CYP17A1, as well as increased expression of enzymes in the steroidogenesis pathway, including AKR1C3 and HSD17B3 (129).
Other androgen synthesis inhibitors are in development at this time, including TAK-700 (Orteronel) and VT-464 (Viamet), both of which are more selective for the 17, 20-lyase inhibition (130). TAK-700 is further in development, currently accruing for another phase III clinical trial, this time assessing efficacy in chemotherapy-naive CRPC patients; the initial phase III study in patients who had been treated with docetaxel demonstrated an improvement in radiographic progression-free survival (HR 0.755), but it did not meet the primary endpoint of improvement in overall survival (HR 0.894) (130).
Enzalutamide and androgen receptor (AR) inhibitors
In response to the many AR mediated mechanisms of resistance found leading to development of CRPC, there has been development of a new generation of androgen-receptor signaling inhibitors. The main agent in this class is enzalutamide (MDV-3100, ENZA, Xtandi), which has been demonstrated to have a multi-pronged approach—preventing testosterone binding to AR, AR nuclear translocation, AR binding to DNA, and co-activator recruitment (106). While the AFFIRM III trial demonstrated a 4.8-month survival benefit over placebo in CRPC patients who had failed docetaxel and the PREVAIL trial demonstrated an overall survival and radiographic progression-free survival over placebo in chemotherapy-naïve CRPC patients (131,132), not all the patients benefited from treatment—a subset of patients continued to progress, indicating that there are significant resistance mechanisms that need to be identified and addressed.
One mechanism by which CRPC develops resistance to enzalutamide, and potentially other treatment modalities, is the process of autophagy. Autophagy is a catabolic process that, besides being constitutively active at a low basal rate, is activated in response to stressors, allowing cells to use lysosomal-mediated degradation of cellular proteins and organelles to regenerate energy (133-135). Autophagy can be used by cancer cells to prolong their survival under harsh conditions of metabolic stress in the tumor microenvironment induced by various treatment modalities, but excessive or deregulated autophagy can push the cells toward autophagic cell death or type-II programmed cell death (136,137). Indeed, androgen deprivation has been shown to induce autophagy, and while the exact mechanism is unknown, suppression of mTOR appears to play a critical role (135,138). Prior studies, by our group and others, have established that administration of autophagy inhibitors, either as monotherapy or in conjunction with established therapies, has had effective cytotoxic result. We demonstrated that the use of clomipramine and metformin, both clinical autophagy inhibitors, significantly increased the cytotoxicity associated with enzalutamide in vitro and in mouse models—the enzalutamide/clomipramine combination decreased tumour size by 91%, compared with a 78% decrease with enzalutamide/metformin (135). There are currently many ongoing clinical trials assessing the role of autophagy inhibitors as concomitant therapy (139), including a study at our institution that has recently been approved to assess metformin and enzalutamide combination therapy.
AR point mutations are also important mechanisms of resistance to enzalutamide, just as in the development of CRPC. The Phe876Leu mutation in the LBD of AR has been reported to make enzalutamide into an agonist of AR, though the clinical relevance of this change has not been documented (140,141). Similar effects were noted for the first generation anti-androgens bicalutamide and flutamide.
Another proposed mechanism is the “glucocorticoid receptor take-over” pathway. Glucocorticoid receptors are nuclear receptors similar in structure to the AR. Glucocorticoids initially have a suppressive effect on prostate cancer, and indeed, are often given in conjunction with early treatments of CRPC, including chemotherapy and abiraterone. However, the DNA binding domain of the glucocorticoid receptor is very similar to the DBD of the AR (142,143), and the glucocorticoid receptor has been shown to bind to many AR regulated genes, suggesting its upregulation in patients treated with chemotherapy or ADT may contribute to enzalutamide resistance (144).
Many of these mechanisms may also affect upcoming androgen-receptor inhibitors in a similar fashion. For example, ARN-509, another novel AR antagonist which is currently in the accrual phase of a multi-center phase III clinical trial, has been shown to be susceptible to the same AR F876L mutation that converts it to an agonist (145). Other agents currently being developed include ODM-201.
Targeting the androgen receptor (AR): the next step in prostate cancer therapy
As recently published in the New England Journal of Medicine, Antonarakis and collaborators demonstrated that 20-40% of circulating tumor cells in CRPC patients treated with abiraterone and enzalutamide have ARV7 constitutively active (146). More importantly, however, they demonstrated in this prospective trial that the subset of men with ARV7 in circulating tumor cells had a significantly lower PSA response rate, shorter progression-free survival and overall survival compared to men without ARV7 expression. This study, our own research (62), and studies by other groups (94,145-147) demonstrate that ARVs are an important mechanism of resistance to newer CRPC agents. Liu et al. demonstrated that AR-V7 was present in a number of prostate cancer cell lines and that it was able to activate the PSA promoter in LNCaP and PC3 cells in the absence of androgen (148). With the loss of the LBD on the AR as seen in ARV-7, CRPC cell lines overcome the loss of circulating and intratumoral androgens mediated by abiraterone. Loss of the LBD, and concurrent ligand-independent binding of AR to ARE’s, is thought to be the underlying mechanism of resistance to enzalutamide. Li et al. demonstrated that knockdown of AR-V limited androgen-independent growth rate of CWR22Rv1 cells and restored responsiveness to anti-androgens (147).
With the growing body of evidence pointing to the important role of ARV’s in the development of resistance and the concurrent finding that many of the current mechanisms of progression to CRPC involve alterations in the AR pathway, targeting the AR appears to be the next major step in prostate cancer therapy.
Our lab previously identified niclosamide, used clinically to treat helminth infections, as an inhibitor of ARV7, by promoting its degradation; co-treatment with enzalutamide demonstrated a synergistic response (148). Similarly, we also established that miR-let-7c, a microRNA of the let-7 family, antagonizes AR expression via c-myc degradation, leading to inhibition of prostate cancer proliferation (149). Others have also started to focus on the AR itself as a target for therapy—either reducing its expression or promoting its degradation. Lai et al. have identified ASC-J9, a novel AR degradation enhancer currently utilized clinically for other pathologies (150). Sadar and colleagues have been focusing on EPI-001, a small molecule that inhibits the N-terminal domain (NTD), which is present on both wild-type AR and AR variants (151).
Perhaps by targeting the AR and its variants, we may be able to overcome the deficiencies of current CRPC treatments.
Conclusions
Prostate cancer, especially locally advanced and metastatic disease, continues to be a burden on the healthcare system. While the prognosis is good for men diagnosed with localized disease, the prognosis remains poor for men with more advanced disease. All current therapies, from ADT to chemotherapy, merely slow the progression of disease, but all patients inevitably progress on therapy. Understanding the mechanisms by which these patients develop resistance to ADT, then subsequently to docetaxel, abiraterone, and enzalutamide, is important to identify future targets of therapy.
Acknowledgements
Funding: This work is supported in part by Grants DOD PC111467 and Medivation/Astellas to CPE, NIH RO1 CA 165263−13 to H-JK and by a Stand Up To Cancer—Prostate Cancer Foundation-Prostate Dream Team Translational Cancer Research Grant SU2C-AACR-PCF DT0812 to Eric Small, Owen Witte and CPE. This research grant is made possible by the generous support of the Movember Foundation. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research: the costs of publication of this article were defrayed.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- Studer UE, Hauri D, Hanselmann S, et al. Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol 2004;22:4109-18. [PubMed]
- Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 2009;6:76-85. [PubMed]
- Hughes IA, Davies JD, Bunch TI, et al. Androgen insensitivity syndrome. Lancet 2012;380:1419-28. [PubMed]
- Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther 2013;140:223-38. [PubMed]
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol 2002;20:3001-15. [PubMed]
- Kim YS, Alarcon SV, Lee S, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem 2009;9:1479-92. [PubMed]
- Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl 2010;12:639-57. [PubMed]
- Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007;28:778-808. [PubMed]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34-45. [PubMed]
- Lindzey J, Kumar MV, Grossman M, et al. Molecular mechanisms of androgen action. Vitam Horm 1994;49:383-432. [PubMed]
- Roy AK, Lavrovsky Y, Song CS, et al. Regulation of androgen action. Vitam Horm 1999;55:309-52. [PubMed]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 2004;67:417-34. [PubMed]
- Niu Y, Altuwaijri S, Yeh S, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A 2008;105:12188-93. [PubMed]
- Yu J, Yu J, Mani RS, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010;17:443-54. [PubMed]
- Green SM, Mostaghel EA, Nelson PS. Androgen action and metabolism in prostate cancer. Mol Cell Endocrinol 2012;360:3-13. [PubMed]
- Geller J, Albert J, Nachtsheim D, et al. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res 1979;33:103-11. [PubMed]
- Mohler JL, Gregory CW, Ford OH 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004;10:440-8. [PubMed]
- Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447-54. [PubMed]
- Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. Embo J 2011;30:2719-33. [PubMed]
- Mostaghel EA. Abiraterone in the treatment of metastatic castration-resistant prostate cancer. Cancer Manag Res 2014;6:39-51. [PubMed]
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972;22:232-40. [PubMed]
- Crawford ED, Hou AH. The role of LHRH antagonists in the treatment of prostate cancer. Oncology (Williston Park) 2009;23:626-30. [PubMed]
- Agarwal N, Hussain M. Management of hormone-sensitive metastatic prostate cancer. Hematol Oncol Clin North Am 2013;27:1221-41. viii. [PubMed]
- Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab 2013;27:603-16. [PubMed]
- Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J 2010;4:380-4. [PubMed]
- Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol 2013;190:429-38. [PubMed]
- Scher HI, Liebertz C, Kelly WK, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 1997;15:2928-38. [PubMed]
- Fosså SD, Slee PH, Brausi M, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol 2001;19:62-71. [PubMed]
- Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol 1993;149:607-9. [PubMed]
- Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer 1999;81:242-51. [PubMed]
- Kumagai J, Hofland J, Erkens-Schulze S, et al. Intratumoral conversion of adrenal androgen precursors drives androgen receptor-activated cell growth in prostate cancer more potently than de novo steroidogenesis. Prostate 2013;73:1636-50. [PubMed]
- Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet 1995;9:401-6. [PubMed]
- Gregory CW, Johnson RT Jr, Mohler JL, et al. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res 2001;61:2892-8. [PubMed]
- Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol 2008;8:440-8. [PubMed]
- Liu W, Xie CC, Zhu Y, et al. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 2008;10:897-907. [PubMed]
- Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11-22. [PubMed]
- Makridakis NM, di Salle E, Reichardt JK. Biochemical and pharmacogenetic dissection of human steroid 5 alpha-reductase type II. Pharmacogenetics 2000;10:407-13. [PubMed]
- Scariano JK, Treat E, Alba F, et al. The SRD5A2 V89L polymorphism is associated with severity of disease in men with early onset prostate cancer. Prostate 2008;68:1798-805. [PubMed]
- Sack JS, Kish KF, Wang C, et al. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci U S A 2001;98:4904-9. [PubMed]
- Suzuki H, Akakura K, Komiya A, et al. Codon 877 mutation in the androgen receptor gene in advanced prostate cancer: relation to antiandrogen withdrawal syndrome. Prostate 1996;29:153-8. [PubMed]
- Steketee K, Timmerman L, Ziel-van der Made AC, et al. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer 2002;100:309-17. [PubMed]
- Dehm SM, Schmidt LJ, Heemers HV, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68:5469-77. [PubMed]
- van de Wijngaart DJ, Molier M, Lusher SJ, et al. Systematic structure-function analysis of androgen receptor Leu701 mutants explains the properties of the prostate cancer mutant L701H. J Biol Chem 2010;285:5097-105. [PubMed]
- Thompson J, Saatcioglu F, Janne OA, et al. Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol 2001;15:923-35. [PubMed]
- Hara T, Kouno J, Nakamura K, et al. Possible role of adaptive mutation in resistance to antiandrogen in prostate cancer cells. Prostate 2005;65:268-75. [PubMed]
- Gottlieb B, Beitel LK, Wu JH, et al. The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat 2004;23:527-33. [PubMed]
- Gottlieb B, Beitel LK, Nadarajah A, et al. The androgen receptor gene mutations database: 2012 update. Hum Mutat 2012;33:887-94. [PubMed]
- Wolf IM, Heitzer MD, Grubisha M, et al. Coactivators and nuclear receptor transactivation. J Cell Biochem 2008;104:1580-6. [PubMed]
- Hermanson O, Glass CK, Rosenfeld MG. Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab 2002;13:55-60. [PubMed]
- Agoulnik IU, Weigel NL. Androgen receptor coactivators and prostate cancer. Adv Exp Med Biol 2008;617:245-55. [PubMed]
- Ni L, Yang CS, Gioeli D, et al. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol 2010;30:1243-53. [PubMed]
- Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 2005;19:631-42. [PubMed]
- Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer 2009;9:615-30. [PubMed]
- Ueda T, Mawji NR, Bruchovsky N, et al. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem 2002;277:38087-94. [PubMed]
- Chung AC, Zhou S, Liao L, et al. Genetic ablation of the amplified-in-breast cancer 1 inhibits spontaneous prostate cancer progression in mice. Cancer Res 2007;67:5965-75. [PubMed]
- Debes JD, Schmidt LJ, Huang H, et al. p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res 2002;62:5632-6. [PubMed]
- Wissmann M, Yin N, Muller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 2007;9:347-53. [PubMed]
- Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009;138:245-56. [PubMed]
- Nadiminty N, Lou W, Lee SO, et al. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci U S A 2006;103:7264-9. [PubMed]
- Nadiminty N, Tummala R, Liu C, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther 2013;12:1629-37. [PubMed]
- Shtivelman E, Beer TM, Evans CP. Molecular pathways and targets in prostate cancer. Oncotarget 2014;5:7217-59. [PubMed]
- Toren P, Zoubeidi A. Targeting the PI3K/Akt pathway in prostate cancer: Challenges and opportunities Int J Oncol 2014;45:1793-801. (Review). [PubMed]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13:140-56. [PubMed]
- Jiang X, Chen S, Asara JM, et al. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J Biol Chem 2010;285:14980-9. [PubMed]
- Xin L, Teitell MA, Lawson DA, et al. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A 2006;103:7789-94. [PubMed]
- Vlaeminck-Guillem V, Gillet G, Rimokh R. SRC: marker or actor in prostate cancer aggressiveness. Front Oncol 2014;4:222. [PubMed]
- Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene 2004;23:7918-27. [PubMed]
- Chang YM, Bai L, Liu S, et al. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene 2008;27:6365-75. [PubMed]
- Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia 2007;9:90-100. [PubMed]
- Lee LF, Guan J, Qiu Y, et al. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol 2001;21:8385-97. [PubMed]
- Lee LF, Louie MC, Desai SJ, et al. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene 2004;23:2197-205. [PubMed]
- Gong J, Zhu J, Goodman OB Jr, et al. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene 2006;25:2011-21. [PubMed]
- Fan S, Gao M, Meng Q, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 2005;24:1749-66. [PubMed]
- Sumitomo M, Shen R, Goldberg JS, et al. Neutral endopeptidase promotes phorbol ester-induced apoptosis in prostate cancer cells by inhibiting neuropeptide-induced protein kinase C delta degradation. Cancer Res 2000;60:6590-6. [PubMed]
- Unni E, Sun S, Nan B, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res 2004;64:7156-68. [PubMed]
- Recchia I, Rucci N, Festuccia C, et al. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer 2003;39:1927-35. [PubMed]
- Lara PN Jr, Longmate J, Evans CP, et al. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs 2009;20:179-84. [PubMed]
- Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013;14:1307-16. [PubMed]
- Guo W, Liu R, Bhardwaj G, et al. Targeting Btk/Etk of prostate cancer cells by a novel dual inhibitor. Cell Death Dis 2014;5:e1409. [PubMed]
- Wen Y, Hu MC, Makino K, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 2000;60:6841-5. [PubMed]
- Chang KH, Ercole CE, Sharifi N. Androgen metabolism in prostate cancer: from molecular mechanisms to clinical consequences. Br J Cancer 2014;111:1249-54. [PubMed]
- Sharifi N. Minireview: Androgen metabolism in castration-resistant prostate cancer. Mol Endocrinol 2013;27:708-14. [PubMed]
- Simard J, Ricketts ML, Gingras S, et al. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev 2005;26:525-82. [PubMed]
- Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2011;108:13728-33. [PubMed]
- Yepuru M, Wu Z, Kulkarni A, et al. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res 2013;19:5613-25. [PubMed]
- Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res 2005;11:4653-7. [PubMed]
- Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815-25. [PubMed]
- Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5 alpha-reductase isozymes. J Biol Chem 1993;268:17404-12. [PubMed]
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem 1994;63:25-61. [PubMed]
- Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 2008;68:6407-15. [PubMed]
- Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 2013;154:1074-84. [PubMed]
- Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer 2011;18:R183-96. [PubMed]
- Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res 2009;69:2305-13. [PubMed]
- Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 2009;69:16-22. [PubMed]
- Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest 2010;120:2715-30. [PubMed]
- Hörnberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One 2011;6:e19059. [PubMed]
- Yang X, Guo Z, Sun F, et al. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem 2011;286:36152-60. [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [PubMed]
- Serpa Neto A, Tobias-Machado M, Kaliks R, et al. Ten years of docetaxel-based therapies in prostate adenocarcinoma: a systematic review and meta-analysis of 2244 patients in 12 randomized clinical trials. Clin Genitourin Cancer 2011;9:115-23. [PubMed]
- Marech I, Vacca A, Ranieri G, et al. Novel strategies in the treatment of castration-resistant prostate cancer Int J Oncol 2012;40:1313-20. (Review). [PubMed]
- Sweeney C, Chen YH, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol 2014;32:abstr LBA2.
- Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A 1973;70:765-8. [PubMed]
- McGrogan BT, Gilmartin B, Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta 2008;1785:96-132.
- Sternberg CN, Petrylak DP, Madan RA, et al. Progress in the treatment of advanced prostate cancer. Am Soc Clin Oncol Educ Book 2014.117-31. [PubMed]
- Fabbri F, Amadori D, Carloni S, et al. Mitotic catastrophe and apoptosis induced by docetaxel in hormone-refractory prostate cancer cells. J Cell Physiol 2008;217:494-501. [PubMed]
- Kramer G, Schwarz S, Hagg M, et al. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer 2006;94:1592-8. [PubMed]
- Kuroda K, Liu H, Kim S, et al. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy. Prostate 2009;69:1579-85. [PubMed]
- O’Neill AJ, Prencipe M, Dowling C, et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer 2011;10:126. [PubMed]
- Sissung TM, Baum CE, Deeken J, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res 2008;14:4543-9. [PubMed]
- Terry S, Ploussard G, Allory Y, et al. Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer 2009;101:951-6. [PubMed]
- Ploussard G, Terry S, Maille P, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res 2010;70:9253-64. [PubMed]
- Hara T, Ushio K, Nishiwaki M, et al. A mutation in beta-tubulin and a sustained dependence on androgen receptor signalling in a newly established docetaxel-resistant prostate cancer cell line. Cell Biol Int 2010;34:177-84. [PubMed]
- Gan L, Wang J, Xu H, et al. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate 2011;71:1158-66. [PubMed]
- Zhu Y, Liu C, Nadiminty N, et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther 2013;12:1829-36. [PubMed]
- Tantivejkul K, Loberg RD, Mawocha SC, et al. PAR1-mediated NFkappaB activation promotes survival of prostate cancer cells through a Bcl-xL-dependent mechanism. J Cell Biochem 2005;96:641-52. [PubMed]
- Domingo-Domenech J, Oliva C, Rovira A, et al. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res 2006;12:5578-86. [PubMed]
- Zhong B, Sallman DA, Gilvary DL, et al. Induction of clusterin by AKT--role in cytoprotection against docetaxel in prostate tumor cells. Mol Cancer Ther 2010;9:1831-41. [PubMed]
- Zhang H, Kim JK, Edwards CA, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol 2005;7:909-15. [PubMed]
- Sowery RD, Hadaschik BA, So AI, et al. Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int 2008;102:389-97. [PubMed]
- Magadoux L, Isambert N, Plenchette S, et al. Emerging targets to monitor and overcome docetaxel resistance in castration resistant prostate cancer Int J Oncol 2014;45:919-28. (review). [PubMed]
- Shukla S, Gupta S. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Clin Cancer Res 2004;10:3169-78. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [PubMed]
- Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 2009;27:3742-8. [PubMed]
- Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 2012;97:507-16. [PubMed]
- Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011;71:6503-13. [PubMed]
- Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 2011;17:5913-25. [PubMed]
- Agarwal N, Di Lorenzo G, Sonpavde G, et al. New agents for prostate cancer. Ann Oncol 2014;25:1700-9. [PubMed]
- Sternberg CN, de Bono JS, Chi KN, et al. Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol 2014;25:429-34. [PubMed]
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33. [PubMed]
- Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069-75. [PubMed]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007;9:1102-9. [PubMed]
- Nguyen HG, Yang JC, Kung HJ, et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene 2014;33:4521-30. [PubMed]
- Leone RD, Amaravadi RK. Autophagy: a targetable linchpin of cancer cell metabolism. Trends Endocrinol Metab 2013;24:209-17. [PubMed]
- Chen N, Karantza V. Autophagy as a therapeutic target in cancer. Cancer Biol Ther 2011;11:157-68. [PubMed]
- Bennett HL, Stockley J, Fleming JT, et al. Does androgen-ablation therapy (AAT) associated autophagy have a pro-survival effect in LNCaP human prostate cancer cells? BJU Int 2013;111:672-82. [PubMed]
- Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol 2014;11:508-16. [PubMed]
- Eisermann K, Wang D, Jing Y, et al. Androgen receptor gene mutation, rearrangement, polymorphism. Transl Androl Urol 2013;2:137-47. [PubMed]
- Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov 2013;3:1030-43. [PubMed]
- Sharifi N. Steroid receptors aplenty in prostate cancer. N Engl J Med 2014;370:970-1. [PubMed]
- Denayer S, Helsen C, Thorrez L, et al. The rules of DNA recognition by the androgen receptor. Mol Endocrinol 2010;24:898-913. [PubMed]
- Claessens F, Helsen C, Prekovic S, et al. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat Rev Urol 2014;11:712-6. [PubMed]
- Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov 2013;3:1020-9. [PubMed]
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. [PubMed]
- Li Y, Chan SC, Brand LJ, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73:483-9. [PubMed]
- Liu C, Lou W, Zhu Y, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res 2014;20:3198-210. [PubMed]
- Nadiminty N, Tummala R, Lou W, et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem 2012;287:1527-37. [PubMed]
- Lai KP, Huang CK, Chang YJ, et al. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am J Pathol 2013;182:460-73. [PubMed]
- Sadar MD. Small molecule inhibitors targeting the “achilles’ heel” of androgen receptor activity. Cancer Res 2011;71:1208-13. [PubMed]


