Prostate cancer metastasis: roles of recruitment and reprogramming, cell signal network and three-dimensional growth characteristics
Introduction
Worldwide, prostate cancer (PCa) ranks third in cancer incidence and sixth in cancer mortality in men (1). PCa has the highest cancer incidence among men in western countries and is the second most common cause of cancer death after lung cancer (2). The American Cancer Association reported that in 2013 PCa accounted for approximately 28% of new cancers diagnosed in the United States (238,590 new cases) with an overall 10% death rate (29,720 cases) (1). While local and regional PCa cases are curable with a 5-year survival of nearly 100%, 5-year survival of PCa patients with metastasis to a distant site goes down to 28% (1), making PCa one of the deadliest cancers. New treatments and diagnostic approaches are badly needed.
The main clinical complication of PCa is bone metastasis. Previous studies comparing progressive castration-resistance and non-metastatic human samples revealed bone lesion metastasis in >80% of all men who die of PCa (3,4). Despite the high occurrence of skeleton metastasis and major patient mortality (5), the molecular mechanisms behind PCa’s prevalence for homing to bone are not well understood. Bone is a dynamic environment, with a balance between bone resorption and bone formation activities. The main players in normal bone remodeling are osteoclast (OC, bone-destructive) and osteoblast (OB, bone-forming) cells (6,7). OC cells express receptor activator of nuclear factor (NF) kappa-B (RANK) and become mature through interaction with their ligand, RANKL, expressed on OB cells located as the membrane-bound form on the bone surface (8,9). The balance between the activities of these cells is controlled by the triad relationship among RANK, RANKL and osteoprotegerin (OPG), a decoy receptor of RANK that binds RANK ligand (RANKL) and blocks RANK-RANKL interaction and subsequent bone resorption (10-12). There are many other players controlling bone development that are also known to affect PCa bone metastasis. The growth factors (GFs) in the bone microenvironment, such as transforming GF-β (TGF-β), bone morphogenetic proteins (BMPs) (13), runt-related transcription factor-2 (RUNX2), stromal cell-derived factor 1 (SDF-1/CXCL12) (14) and endothelin-1 (ET1) have roles in bone turnover and cancer metastasis to bone (15). Some of the main factors involved in normal bone remodeling are secreted by cancer cells (16-21) and could serve as predictors of cancer bone metastasis (22). For instance, chemotaxis of cancer cells toward bone sites could be explained in part by the interaction of CXCL12, a soluble stroma-derived factor, and its receptor CXCR4 on the surface of cancer cells (21,23). It has been shown that cancer cells with bone-homing propensity expressed bone-forming factors such as BMP, insulin-like growth factors (IGF-1) and ET1, all of which affect the balance of bone resorption and formation (24). Cancer cells can also secrete soluble factors, RANKL, parathyroid hormone-related protein (PHTrP) (25) and the interleukin (IL)-6 (26-28) to promote bone resorption. Consequently, higher bone resorption increases bone breakdown that releases TGF-β, IGF-1, and BMPs, which further promote and enhance cancer cell proliferation and survival (29). Taken together, tumor cells interact with bone cells by establishing a “vicious cycle” through secreted soluble factors in the bone microenvironment that culminate in enhanced bone metastasis (30).
In this review, we emphasize three areas. (I) We present a new concept based on our finding that a selective population of cancer cells can recruit and reprogram gene expression and cell behaviors of bystander or dormant cells (DCs), conferring their metastatic potential to bone and soft tissues. (II) We discuss how cell signaling networks are activated by GFs, extracellular matrices (ECMs) and androgen receptors (ARs) to govern the metastatic cascade of cancer cells. (III) We summarize cell and animal model systems used to study PCa progression, share our experience with 3-D culture, and discuss how these culture methods could be further expanded to understand the underlying molecular basis of tumor-microenvironment interaction.
Possible mechanisms of tumor metastasis: recruitment and reprogramming
Tumors are not single insular entities; they are complex tissues composed of various distinct cell types that engage in collaborative interactions with one another during tumorigenesis (31,32). Tumor microenvironments shaped by the presence of tumor cells are therefore critical to allow cooperative interaction among tumor cells and their neighboring cells via soluble factors and ECMs, in order to sustain tumor growth, invasion and metastasis. It is increasingly evident that malignant cancer cells are capable of recruiting and transforming normal or non-tumorigenic bystander cells to serve as active collaborators and participate in tumorigenesis, evade immune surveillance and develop distant metastasis (32-35). The supportive cells recruited to the tumor microenvironment include a heterogeneous population of stromal cells, including fibroblasts, immune cells, endothelial cells, and bone marrow-derived mesenchymal stem and progenitor cells (MSCs) (36). The tumor-stroma crosstalk, either by direct cell-cell interaction or by secreted soluble factors or insoluble ECMs, is reciprocal. Through the interaction of tumor cells with their microenvironments, heterotypic signaling between the diverse cellular constituents of the tumor microenvironment promotes tumor growth, survival and metastatic progression (32) (Figure 1). Investigation of the interactions between cancer cells and their supporting “co-conspirators” during tumorigenesis and metastasis provides a crucial understanding of cancer pathogenesis, leading to the development of novel and effective therapeutic strategies.
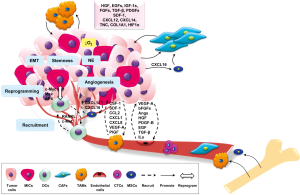
Cancer-associated fibroblastic cells
Since fibroblasts are associated with wound healing, tumors with aberrant wound healing and tissue repair mechanisms were found to recruit a variety of fibroblastic cells. In particular, both local and bone marrow derived MSCs are recruited and activated to become carcinoma-associated fibroblasts (CAFs) or myofibroblasts (34), characterized by the production of α smooth muscle actin (αSMA) (37). Different CAF subpopulations can contribute to a variety of tumor-promoting and differentiation functions with the production of different stimulatory or differentiation factors, whose functions are significantly influenced by the adjacent heterogeneous tumor epithelial cells and other tumor-associated cellular compartments (38). CAFs within prostate tumors secrete high levels of mitogenic GFs and chemokines, including hepatocyte growth factor (HGF), EGF family members, IGF-1s, fibroblast growth factors (FGFs), TGF-β, SDF-1/CXCL12, CXCL14, tenascin-C, collagen 1, and hypoxia inducible factor 1 (HIF1α) that together stimulate cell proliferation and promote epithelial-to-mesenchyme-transition (EMT) (39-42). One of the potential mechanisms underlying PCa progression and metastasis by the recruitment of MSCs is mediated by the CXCL16-CXCR6 signaling axis as recently demonstrated by Jung et al. They found that tumor-derived CXCL16 recruits and interacts with CXCR6-positive MSCs, leading to the transformation of MSCs to CAFs, which produce high levels of SDF-1/CXCL12, further promoting EMT progression and metastasis by up-regulation of CXCR4 in PCa cells (43).
Infiltrating immune cells
The relationship between inflammation and tumorigenesis was first proposed in 1,863 by Rudolf Virchow based on the observation of unusual numbers of infiltrating leukocytes in neoplastic tissues (44). Since then, a plethora of studies have documented that human tumors are infiltrated with heterogeneous populations of inflammatory cells (44-46), in particular macrophages, or tumor-associated macrophages (TAMs). TAMs are derived from monocytes actively recruited to tumors from the blood in response to chemokine cues in the tumor microenvironment (47,48). TAMs can constitute up to 50% of the tumor mass and the high numbers of TAMs present in solid tumors are strongly implicated in cancer progression and metastasis (36,49,50) and correlate with poor patient prognosis (51). TAMs have multifaceted roles; even though TAMs recruited to tumor sites represent the first line of defense as part of an innate immune response, it is well documented that TAMs also support multiple aspects of tumor progression (36,52,53). TAMs produce high levels of reactive oxygen and nitrogen species that can induce DNA damage and mutations of the surrounding epithelium (54). TAMs also secrete several GFs, such as HGF, FGFs, EGF, platelet-derived growth factor (PDGF), and TGF-β that are capable of promoting tumor growth and EMT, leading to metastatic progression (55). Colony-stimulating factor 1 (CSF-1) is one of the key factors that promotes monocyte recruitment and macrophage differentiation, survival, and proliferation (36,51). In a breast cancer model, CSF-1 deficient mice have impaired macrophage differentiation and survival, leading to significantly lower incidence of tumor formation and metastasis (54). Further studies have identified other chemokines, such as CCL2 (56,57), CX3CL1 (58), CXCL8, and SDF-1 (57), and GFs such as vascular endothelial GF A (VEGF-A) and placental GF (PIGF) (59,60) that are expressed by tumor cells, fibroblasts, endothelial cells, or TAMs that can induce monocyte/macrophage recruitment into specific tumor microenvironments (36). Furthermore, TAMs tend to accumulated in the hypoxic regions of tumors, mediated by the chemoattractants endothelin-2 and VEGF regulated by hypoxia-inducible factor-1 (HIF-1) (47,60). TAM recruitment and accumulation in hypoxia regions results in angiogenesis and the progression of tumor cells to a more invasive phenotype (60).
Endothelial cells
Many different cytokines and GFs produced by tumor cells, infiltrating immune cells, and fibroblasts are involved in the recruitment of endothelial cells during angiogenesis, including VEGF, basic FGF (bFGF), angiopoietins, HGF, PDGF-B, EGF, TGF-β, and interleukins (61). These proangiogenic factors can further activate matrix metalloproteases such as matrix metalloproteinase (MMP)-1 and MMP-9 and urokinase-type plasminogen activators (uPA) to break down ECMs and facilitate endothelial cell and pericyte invasion (62) and vascular remodeling (36,63). Recently, Png et al. demonstrated that tumor suppressor miR-126, a miRNA silenced in a variety of common human cancers, non-cell-autonomously suppresses endothelial cell recruitment, metastatic angiogenesis, and metastatic colonization of breast cancer cells in vitro and in vivo. It coordinates the targeting of insulin-like GF binding protein 2 (IGFBP2), phosphatidylinositol transfer protein, cytoplasmic 1 and c-Mer tyrosine kinase, which are novel pro-angiogenic genes and biomarkers of human cancer metastasis (64). Endothelial cells provide blood flow to tumors and they also clearly signal and facilitate cancer cells to metastasize and colonize at distant sites.
Non-tumorigenic and -metastatic bystander and DCs
Using the RANKL-overexpressing PCa cell model, we identified a population of metastasis-initiating cells (MICs) that can recruit bystander non-metastatic cancer cells, in this case red fluorescent protein (RFP)-tagged LNCaP cells, to metastatic sites to participate in skeletal and soft tissue metastasis (65). We also observed that the bystander non-metastatic cancer cells (65,66) can be reprogrammed by the MICs to undergo cytogenetic and gene expression changes, subsequently displaying MIC phenotypes such as EMT, stem cell, neuroendocrine cell and bone-like properties through transactivation of c-Myc/Max and AP4 via RANK-mediated signaling, thereby gaining the ability to migrate, invade, and metastasize to bone and soft tissues (33). Similarly, we found that these genetically modified MICs can recruit and reprogram DCs established from primary PCa tumor tissue and also human circulating tumor cells (hCTCs) freshly obtained from patients by ex vivo culture under a 3-D organoid co-culture system. In this setting, DCs and hCTCs were reprogrammed by the experimental MICs to express elevated genes related to an increased RANKL-RANK signaling. In another study, we observed that experimental PCa epithelial cells can transform normal fibroblastic cells to gain extensive and consistent cytogenetic changes notably found in tumor cells (67). Recently, we extended our work to define two naturally occurring MICs (nMICs) that were isolated by ex vivo culture of the ascites fluid of a bone metastatic PCa patient (68-70), expressing MIC signature genes and displaying MIC properties, including aggressively forming metastatic bone tumors when inoculated intracardially in mice. We demonstrated that, similar to the RANKL-overexpression genetic model of MICs, the nMICs, are capable of recruiting and promoting the growth of DCs in 3-D organoid co-culture. These recruited DCs were shown to be reprogrammed to mimic nMICs with enhanced expression of RANKL-RANK downstream target genes, including the mesenchymal, the stem cells, the neuroendocrine and the osteomimicry biomarkers. We believe CTCs and DCs once recruited and reprogrammed by MICs undergo permanent changes sustained possibly by genetic mutation, cytogenetic changes (e.g., chromosomal rearrangement and translocations) (35) or epigenetic modification by methylation of specific promoters whose status may be controlled by the reciprocal cellular interaction between nMICs and CTCs or DCs (71-75). Understanding the underlying mechanisms of the recruitment and reprograming of DCs or CTCs by either experimental MICs or nMICs will provide significant insights into cancer evolution, progression, and metastasis with major implications for both basic biology and clinical medicine.
Intracellular cell signaling pathways governing the metastatic cascade
Cytokines also have strong pro-tumorigenic activities on host cells in the tumor microenvironment. For example, RANKL exerts multiple effects on bone turnover and metabolism, stem cell renewal, tumor cell proliferation and survival, angiogenesis, and inflammation. These effects are mediated by several common intracellular signaling pathways. We highlight a few examples of specific pathways that are induced by cytokines or their receptors, with emphasis on pathways that offer possibility as potential therapeutic targets (Figure 2).
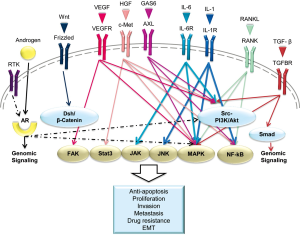
Androgen receptor (AR)
Molecular modeling of PCa has demonstrated that androgens have critical roles in PCa development and progression at all stages of disease, with their actions mediated by AR (76). AR, a large ~110 kDa transcription factor of the steroid nuclear receptor family, can be activated in target organs in a ligand-dependent or -independent manner to support the growth and differentiation of the normal prostate gland as well as the malignant growth and progression of PCa (76). The AR signaling axis is intricately regulated by its interactive factors and converges with a large number of other signaling pathways (77,78). For example, AR was shown to be responsible for metastatic progression of PCa through downstream signal convergence with chemokine receptor/ligand function and G-protein coupled receptors (79). Altered AR functions were observed in cells that expressed tumor-associated AR cofactors (e.g., FOXA1) (80), or formation of AR-dependent gene fusions (e.g., TMPRSS2-ERG) (81) and downstream effectors (e.g., SOX9) (82). AR mutations at the c-terminal ligand-binding domain (83) or deletion of the c-terminal domain to yield truncated AR variants (ARs) (84) have been shown to produce, respectively, promiscuous AR that can be activated by a broad spectrum of steroidal and non-steroidal ligands or a constitutively activated AR without the requirement of steroid ligands (85). AR mutations and ARs have been implicated in the development of CRPC and the resistance of PCa to current androgen antagonist therapies such as enzalutamide or abiraterone acetate (86,87).
RANK ligand (RANKL)
RANKL-RANK signaling has many crucial physiological roles in bone and peripheral soft tissues. Aberrant RANKL–RANK signaling in cancer and bone cells affects cancer bone colonization (87,88). Our group previously reported that RANKL-expressing PCa cells can recruit and reprogram neighboring non-metastatic bystander or DCs via specifically activating transcription factors through the RANK-mediated downstream signaling network in vitro and in vivo (65). Significantly, RANKL and its downstream signaling network in primary human PCa tissues predict patient survival (89). There are two TRAF6-mediated intracellular cascades induced by RANKL-RANK signaling: the classic and non-canonical NF-κB pathways (90,91) and the c-Src-mediated Akt and MAPK protein kinases pathways, enhancing cancer cell migration and survival (92-94).
The interleukin (IL) family
IL-1 and IL-6 activate OBs and OCs, and disrupt the homeostatic balance that controls bone formation and resorption.
IL-1
One of the IL-1 receptors, IL-1β, was recently shown to be overexpressed in non-metastatic cancer cells to promote the growth of large skeletal lesions in mice, whereas the knockdown of IL-1β significantly impaired the bone metastatic progression of a highly metastatic cancer cell line (95). Human PCa tissue specimens isolated from skeletal metastatic patients, while not expressing PSA, were positive for both IL-1β and synaptophysin, a neuroendocrine differentiation marker (95). Like RANKL, IL-1 also initiates a TRAF6-mediated signaling cascade to activate the downstream NF-κB-, JNK-, and MAPK-mediated signaling pathways (96,97).
IL-6
It has been reported that human PCa cells produce IL-6 and that serum levels of IL-6 are elevated in patients with PCa, including serum isolated from patients with advanced hormone-refractory disease. IL-6 has been suggested as a prostate exocrine gene product and a candidate mediator of PCa intra- and inter-cellular communication (98). Binding of IL-6 to its receptor, IL-6R, activates the JAK kinase family (JAK1, JAK2, and TYK2) (99). These kinases phosphorylate STAT-3, promoting its nuclear translocation and transcriptional function (100). IL-6 also activates Ras and promotes its translocation to the plasma membrane where it activates the Raf-MEK pathway. Finally, a third pathway activated by IL-6 is the PI3K-Akt pathway (101,102).
The Wnt pathway
In PCa bone metastasis, Wnt production stimulates osteoblast differentiation and exerts autocrine effects on cancer proliferation (103). Wnt binds to cell surface receptors of the Frizzled family, and activates the members of the Dishevelled family of proteins, such as Dickkopf-related protein 1 (DKK1). The Wnt/Frizzled family subsequently stabilizes β-catenin, which then translocates to the nucleus and promotes multiple downstream signaling to promote bone formation. Wnt/β-catenin signaling also induces OB differentiation through BMP-dependent and -independent signaling pathways. Wnt-mediated signaling also plays a role in the development of invasive CRPC in both mouse and man; Wnt was found to be upregulated in prostate stromal cells when TGFβ, signaling was attenuated (104). The activity of Wnt is controlled by soluble extracellular antagonists including secreted Frizzled-related proteins, WIF-1, Cerberus, and DKK1. This identifies Wnt as a potential therapeutic target to interrupt both autocrine and paracrine interactions between PCa cells and the interactions between PCa-OB and PCa-stromal cells (105).
TGF-β-mediated pathway
TGF-β plays a tumor suppressive role in normal and pre-neoplastic epithelia, but paradoxically promotes motility and resistance to cell death in transformed epithelia (106). Activated TGF-β signaling induces EMT in PCa, suppresses host immune surveillance and drives cancer metastasis (107). Increased TGF-β1 expression by tumor cells also correlates with tumor progression in lung, colorectal, and gastric cancers (108). Mechanistically, TGF-β binds to heterodimeric receptors (type I and type II TGF-β receptors) and can activate the canonical Smads signaling pathway or Smad-independent pathways through PI3K, MAPK, and Akt (109). TGF-β mediates both autocrine and paracrine signaling during PCa progression.
Receptor of tyrosine kinase family
Although receptor tyrosine kinases (RTKs) are important in normal physiology, dysregulation of some RTKs has been implicated in tumor development and progression (110). Because of cross-talk between RTKs, it has redundant functions and can converge with common downstream cell signaling networks, making it difficult to target independently without the concerns of off-target effects. Cancer cells can acquire RTKs resistance after treatment. Therefore, development of inhibitors for multiple RTKs is an active area of pursuit.
c-Met
The expression of c-Met and its ligand, HGF, correlate with PCa metastasis and disease recurrence, with the highest c-Met levels in bone metastases compared with soft tissue and lymph node metastases (111,112). The major signaling networks linking HGF/c-Met are the MAPK, Akt, STAT-3, RANKL-RANK, and NF-κB signaling cascades (113-116).
VEGF receptors (VEGFR)
VEGF and its receptor (VEGFR) are well known to be potent stimulators of angiogenesis in both physiological and pathological conditions and are highly expressed in most solid tumors, including PCa (117). Like other RTKs, VEGF/VEGFR can trigger the MAPK and Akt-dependent axes for proliferation and survival (118,119). VEGF has also been shown to activate FAK and associated proteins for maintenance of survival signals in endothelial cells (119,120).
Axl
Axl has recently been identified as a critical factor driving tumor cell invasion, migration, pro-inflammatory cytokine production, anti-apoptosis, and proliferation. The ligand growth arrest specific gene-6 (GAS6) is the only known ligand for Axl (121). GAS6/Axl activates the Akt pathway to protect cells from apoptosis via multiple mechanisms. In particular, Akt activates the mTOR pathway, inhibits pro-apoptotic caspase 3, and phosphorylates NF-κB, which up-regulates the anti-apoptotic proteins Bcl-2 and Bcl-xL (122,123). In some cell types, Axl also activates the MAPK pathway and contributes to cancer invasion (124).
In summary, this section reviewed selected cell signaling network driving by soluble factors and their receptors that governs PCa growth, invasion and metastasis. Data collected in this section are derived from cell lines, animal models and some are confirmed by clinical specimens. The utility to target effectively cell signaling network and its translation await additional future studies and critical analyses of experimental data collected thus far by systems biology methods to overcome potential converging and redundant cell signaling pathways that hinder cancer cure at the bedside.
Current PCa models and their limitations
Human PCa cell lines
Extensive use of immortalized cell lines has greatly increased our understanding of the biology of PCa and its development through gene deregulation. In PCa research, utilizing cell lines have given us an understanding of crucial molecular mechanisms that lead to bone and soft tissue metastases such as PCa androgen-independent progression (69,125-127), the roles of ARs and their ARs in promoting cell signaling networks in the castration-resistant state (128) and the convergence of cell signaling pathways that explains how cancer cells gain therapeutic resistance (129-131). A detailed characterization of PCa cell lines was reviewed by Sobel et al. (132) and Russell et al. (133).
Despite the knowledge we have gained about PCa using 2-D tissue culture, many disadvantages limit the extent of our understanding and more cell lines with diverse phenotypes are needed. Since most PCa cell lines are derived from a metastatic site, new cell lines from early carcinogenic events could help us to understand the initial steps of PCa transformation and progression from normal tissues. Furthermore, PCa is a heterogeneous disease, samples from same person or different locations in the metastatic cascade were found to behave differently. MDA PCa 2a and 2b, for instance, were derived from bone metastatic specimen of one patient, yet their morphologies and behaviors are quite different (134,135). Therefore, studying different aspects of PCa in the limited number of available cell lines can address only in part the complexity of the disease. More importantly, current monolayer cultures also recapitulate in part the vital PCa cellular interactions with cellular factors including epithelial and stromal cells or non-cellular factors such as ECMs within the context of the tumor microenvironment.
Mouse models of PCa
Given the limitations of tissue culture, in vivo models are often used to better mimic the natural history and the complexity of this disease. Mouse models in PCa research include xenograft and patient-derived xenograft models and genetically engineered models (GEM), detailed reviewed by Ittmann et al. (136) and Valkenburg and Williams (137). In addition, Goldstein and Witte (138) and Shen and Abate-Shen (139) emphasized the importance of tumor microenvironment interactions that promote PCa development in murine models. Mice are valuable models in PCa biology because, like humans, they are susceptible to cancer development when introduced with oncogenes or carcinogens. Mouse genome shares 95% homology to the human genome and they are relatively easy to manipulate genetically (140,141). However, mouse models have distinct limitations. First and foremost, the mouse and human anatomy are different. Unlike the human prostate, the mouse prostate has four lobes (142) and no clear analog has been delineated between mouse prostate lobes and the human peripheral zone where human PCa arises. In addition to anatomic dissimilarity and the size difference between human and mice prostate glands, dietary, hormonal, age and strain, and gene-environment interactions need to be considered when a mouse model is being used to understand histopathologic changes, etiologic and environmental factors and drug screening studies in human PCa (140,143). Some of the main concerns about using mouse models are: (I) spontaneous PCa in mice is very uncommon and distant metastasis to bone is even more rare, despite forced genetic manipulation (47); (II) age is the main known risk factor for PCa and mice have a 30-50 times shorter lifespan than humans (144); and (III) the most-used mouse models are immune compromised. This combination of drawbacks presents formidable challenges when analyzing mouse models of human PCa (136). To study human immune and drug responses to cancer cell growth, conventional nude and severe combined immunodeficiency (SCID) mouse models are being replaced by immunodeficient mice bearing a mutated IL-2 receptor gamma chain. These highly immunodeficient mice, NOG (145) and NSG (146), allow development of human immune systems, including T and B cells through hematopoietic stem cell transplantation. These models successfully permit in vivo investigation of the human immune response to primary human cancer and malignant MSCs (147).
Three-dimensional (3-D) in vitro models to mimic PCa progression and metastasis
Over time, it has become evident that PCa is not a single cell disease. Besides the heterogeneity within PCa tumors, there are many factors, including the contributory roles of host microenvironment stromal cells, immune cells, endothelial cells, growth regulatory factors and ECMs that play crucial roles regulating PCa progression and ultimate metastatic behavior (148,149). So far the best way to study and recapitulate these interactions is the use of animal models though as discussed above, animal models also have their own limitations. An alternative approach is the use of in vitro 3-D models which provide simplified and more economical systems to mimic tumor-host microenvironment interactions in vivo (150-156). Under 3-D growth conditions, tissues, cells, GFs and ECM scan are prepared as physical scaffolding for defined biomechanical strength and composition resembling the pathophysiologic conditions of tumors in vivo. With respect to ECM, 3-D growth provides physical support of tissues and cells and mediates biological and physical cues from the cell external to various pathophysiological commands, which results in changes in cell morphology (157), proliferation, survival (158), migration and adhesion (159,160) through interaction of cell surface α- and β-integrin subunits to specific ECMs (161,162). Dimerization of each αβ subunit on cells dictates binding to specific ECM molecules and determines the activation of downstream signaling and ultimately cell fate (163).
Besides ECM’s direct effect on cell behavior, mainly through integrins, ECM also has a significant role in regulating cell surface GF signaling (164). ECM has binding sites for GFs such as FGFs and VEGFs (165) and they can be cleaved and released as soluble factors under certain pathophysiologic conditions. Additionally, integrins on the cell surface can also contribute to GF activation or degradation. For instance, a study of epidermoid carcinoma has shown that α2β1 integrin co-localization with EGFR is required for further activation of Akt and Rho GTPases (166). In a separate study, Caswell et al. showed that α5β1 integrin can form a complex with EGFR1. Consequently, the membrane bound complex regulates protein kinase B (PKB) signaling and enhances cancer invasion (167). Figure 3 illustrates common known signaling pathways enhancing PCa bone metastasis that are stimulated by ECMs.
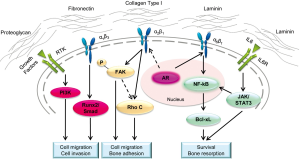
Given the broad influence of ECM in cell biological functions, ECM protein abnormality potentially leads to various human diseases related to skeleton development and remodeling, stem cell differentiation, and inflammation, such as multiple sclerosis or osteoarthritis as well as cancer initiation and progression (174-176). Therefore, the detailed study of the role of ECM in cancer progression and metastasis is crucial and requires proper models.
Since cells grown in 2-D and 3-D cultures differ in their physical contacts with ECM scaffolds, and biological and behavioral changes caused by cell-cell and cell-ECM interaction in the microenvironment, the importance of 3-D cultures in cancer biology studies is now well accepted (176-179). Wang et al. compared the growth of non-malignant HMT-3522 breast cells with those of the malignant HMT-3522 subline and documented the critical functional differences of β1 integrin in breast cancer cells. They showed that in cells grown as 3-D, but not 2-D, anti-β1 integrin antibody treatment restored malignant but not non-malignant breast cancer cells to normalcy (180). In regard to PCa, published studies show restored AR expression in the PC3 and LNCaPRANKL cell lines after growth in matrigel and 3-D suspension culture, respectively (169,181). These results raise the question of whether 3-D culture and co-culture models mimic better the pathophysiology of PCa when considering the screening of AR antagonists against the growth of CRPC.
3-D models were utilized for the first time by Miller in 1985, who studied the drug resistance of mouse mammary tumors in a 3-D collagen gel culture (182). Since then, different types of 3-D models have been developed and many improvements have been made. Current 3D models can be classified into: (I) spontaneous cell aggregation; (II) matrix-embedded cells; and (III) tissue-engineered scaffolds. A detailed discussion of the available 3-D models is beyond the scope of this review. We have summarized the pros and cons of the most commonly used 3-D models in Table 1.
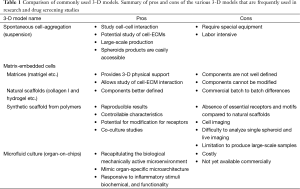
Full table
Despite the advantages of 3-D over 2-D models in mimicking the growth of cancer in vivo, there remain technical difficulties that need to overcome. These include capturing high resolution 3-D imaging and real-time cell tracking. Also, with matrix-embedded cells there is limited access to the cells for DNA/RNA, protein and immunoassay studies. The available 3-D models and techniques are rapidly improving through multidisciplinary collaborations between biologists and biomaterial engineers, and many limitations have been resolved. For example, Gao et al. (183) employed improved GF reduced Matrigel as scaffold for 3-D organoid culture and have successfully established long term culture of freshly harvested human PCa and CTCs. More detailed studies and further optimization are required to develop reliable 3-D models with reproducible data before they can be widely used to bridge the gap between the traditional 2-D cultures and the complex in vivo models.
Acknowledgments
This work was supported financially in part by NCI grants 2P01CA098912 and 1R01CA122602, a Board of Governors Cancer Research Chair, a Spielberg Family Foundation grant, a Prostate Cancer Foundation Challenge grant (LW Chung), and a Prostate Cancer Foundation Young Investigator Award (GC Chu). The authors also acknowledge Dr. Ruoxiang Wang and Mr. Gary Mawyer for editorial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2013. Available online: .http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf
- Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer 2004;101:2371-490. [PubMed]
- Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000;31:578-83. [PubMed]
- Harada M, Iida M, Yamaguchi M, et al. Analysis of bone metastasis of prostatic adenocarcinoma in 137 autopsy cases. Adv Exp Med Biol 1992;324:173-82. [PubMed]
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [PubMed]
- Boyce BF, Yoneda T, Guise TA. Factors regulating the growth of metastatic cancer in bone. Endocr Relat Cancer 1999;6:333-47. [PubMed]
- Zaidi M. Skeletal remodeling in health and disease. Nat Med 2007;13:791-801. [PubMed]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337-42. [PubMed]
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165-76. [PubMed]
- Honore P, Luger NM, Sabino MA, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000;6:521-8. [PubMed]
- Hsu H, Lacey DL, Dunstan CR, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A 1999;96:3540-5. [PubMed]
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309-19. [PubMed]
- Mundy GR, Chen D, Zhao M, et al. Growth regulatory factors and bone. Rev Endocr Metab Disord 2001;2:105-15. [PubMed]
- Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999;283:845-8. [PubMed]
- Takuwa Y, Masaki T, Yamashita K. The effects of the endothelin family peptides on cultured osteoblastic cells from rat calvariae. Biochem Biophys Res Commun 1990;170:998-1005. [PubMed]
- Dallas SL, Miyazono K, Skerry TM, et al. Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J Cell Biol 1995;131:539-49. [PubMed]
- Killian CS, Corral DA, Kawinski E, et al. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem Biophys Res Commun 1993;192:940-7. [PubMed]
- Mohammad KS, Guise TA. Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res 2003.S67-74. [PubMed]
- Nadiminty N, Lou W, Lee SO, et al. Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clin Cancer Res 2006;12:1420-30. [PubMed]
- Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem 2003;89:462-73. [PubMed]
- Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002;62:1832-7. [PubMed]
- Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537-49. [PubMed]
- Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res 2005;20:318-29. [PubMed]
- Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun 2005;328:679-87. [PubMed]
- Powell GJ, Southby J, Danks JA, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res 1991;51:3059-61. [PubMed]
- Basolo F, Fiore L, Fontanini G, et al. Expression of and response to interleukin 6 (IL6) in human mammary tumors. Cancer Res 1996;56:3118-22. [PubMed]
- Blay JY, Schemann S, Favrot MC. Local production of interleukin 6 by renal adenocarcinoma in vivo. J Natl Cancer Inst 1994;86:238. [PubMed]
- Thabard W, Collette M, Mellerin MP, et al. IL-6 upregulates its own receptor on some human myeloma cell lines. Cytokine 2001;14:352-6. [PubMed]
- Yin JJ, Selander K, Chirgwin JM, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999;103:197-206. [PubMed]
- Guise TA, Mundy GR. Cancer and bone. Endocr Rev 1998;19:18-54. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Chu GC, Chung LW. RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev. 2014;33:497-509. [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [PubMed]
- Rhee HW, Zhau HE, Pathak S, et al. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev Biol Anim 2001;37:127-40. [PubMed]
- Wels J, Kaplan RN, Rafii S, et al. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev 2008;22:559-74. [PubMed]
- Paunescu V, Bojin FM, Tatu CA, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med 2011;15:635-46. [PubMed]
- Hägglöf C, Bergh A. The stroma-a key regulator in prostate function and malignancy. Cancers (Basel) 2012;4:531-48. [PubMed]
- Erez N, Truitt M, Olson P, et al. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010;17:135-47. [PubMed]
- Franco OE, Shaw AK, Strand DW, et al. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 2010;21:33-9. [PubMed]
- Orr B, Riddick AC, Stewart GD, et al. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene 2012;31:1130-42. [PubMed]
- Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev 2009;19:67-73. [PubMed]
- Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 2013;4:1795. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [PubMed]
- Mihm MC Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996;74:43-7. [PubMed]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904-12. [PubMed]
- Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31. [PubMed]
- Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996;382:635-8. [PubMed]
- Kelly PM, Davison RS, Bliss E, et al. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer 1988;57:174-7. [PubMed]
- Leek RD, Harris AL, Lewis CE. Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukoc Biol 1994;56:423-35. [PubMed]
- Lee HW, Choi HJ, Ha SJ, et al. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta 2013;1835:170-9.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [PubMed]
- Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193:727-40. [PubMed]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71-8. [PubMed]
- Ansari AW, Heiken H, Meyer-Olson D, et al. CCL2: a potential prognostic marker and target of anti-inflammatory strategy in HIV/AIDS pathogenesis. Eur J Immunol 2011;41:3412-8. [PubMed]
- Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549-55. [PubMed]
- Ancuta P, Moses A, Gabuzda D. Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology 2004;209:11-20. [PubMed]
- Goswami S, Sahai E, Wyckoff JB, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 2005;65:5278-83. [PubMed]
- Nakao S, Kuwano T, Tsutsumi-Miyahara C, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest 2005;115:2979-91. [PubMed]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 2007;100:782-94. [PubMed]
- Chantrain CF, Henriet P, Jodele S, et al. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer 2006;42:310-8. [PubMed]
- Hildenbrand R, Dilger I, Horlin A, et al. Urokinase and macrophages in tumour angiogenesis. Br J Cancer 1995;72:818-23. [PubMed]
- Png KJ, Halberg N, Yoshida M, et al. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2012;481:190-4. [PubMed]
- Chu GC, Zhau HE, Wang R, et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr Relat Cancer 2014;21:311-26. [PubMed]
- Hu P, Chu GC, Zhu G, et al. Multiplexed quantum dot labeling of activated c-Met signaling in castration-resistant human prostate cancer. PLoS One 2011;6:e28670. [PubMed]
- Pathak S, Nemeth MA, Multani AS, et al. Can cancer cells transform normal host cells into malignant cells? Br J Cancer 1997;76:1134-8. [PubMed]
- Chung LW, Zhau HE, Wu TT. Development of human prostate cancer models for chemoprevention and experimental therapeutics studies. J Cell Biochem Suppl 1997;28-29:174-81. [PubMed]
- Zhau HE, Odero-Marah V, Lue HW, et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis 2008;25:601-10. [PubMed]
- Zhau HY, Chang SM, Chen BQ, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A 1996;93:15152-7. [PubMed]
- Delaval K, Feil R. Epigenetic regulation of mammalian genomic imprinting. Curr Opin Genet Dev 2004;14:188-95. [PubMed]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science 2001;293:1068-70. [PubMed]
- Kim KC, Friso S, Choi SW. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem 2009;20:917-26. [PubMed]
- Steenman MJ, Rainier S, Dobry CJ, et al. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet 1994;7:433-9. [PubMed]
- Vu TH, Hoffman AR. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature 1994;371:714-7. [PubMed]
- Cunha GR, Ricke W, Thomson A, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 2004;92:221-36. [PubMed]
- Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 2010;21:315-24. [PubMed]
- Yuan X, Cai C, Chen S, et al. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene 2014;33:2815-25. [PubMed]
- Furusato B, Mohamed A, Uhlen M, et al. CXCR4 and cancer. Pathol Int 2010;60:497-505. [PubMed]
- Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009;138:245-56. [PubMed]
- Udager AM, Shi Y, Tomlins SA, et al. Frequent discordance between ERG gene rearrangement and ERG protein expression in a rapid autopsy cohort of patients with lethal, metastatic, castration-resistant prostate cancer. Prostate 2014;74:1199-208. [PubMed]
- Thomsen MK, Butler CM, Shen MM, et al. Sox9 is required for prostate development. Dev Biol 2008;316:302-11. [PubMed]
- Duff J, McEwan IJ. Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Mol Endocrinol 2005;19:2943-54. [PubMed]
- Yang X, Guo Z, Sun F, et al. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem 2011;286:36152-60. [PubMed]
- Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:12-23. [PubMed]
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. [PubMed]
- Hanada R, Leibbrandt A, Hanada T, et al. Central control of fever and female body temperature by RANKL/RANK. Nature 2009;462:505-9. [PubMed]
- Dougall WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 2012;18:326-35. [PubMed]
- Hu Y, Ek-Rylander B, Wendel M, et al. Reciprocal effects of Interferon-γ and IL-4 on differentiation to osteoclast-like cells by RANKL or LPS. Oral Dis 2014;20:682-92. [PubMed]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004;25:280-8. [PubMed]
- Dickson KM, Bhakar AL, Barker PA. TRAF6-dependent NF-kB transcriptional activity during mouse development. Dev Dyn 2004;231:122-7. [PubMed]
- Kim HH, Shin HS, Kwak HJ, et al. RANKL regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. FASEB J 2003;17:2163-5. [PubMed]
- Tang ZN, Zhang F, Tang P, et al. RANKL-induced migration of MDA-MB-231 human breast cancer cells via Src and MAPK activation. Oncol Rep 2011;26:1243-50. [PubMed]
- Zhang L, Teng Y, Zhang Y, et al. C-Src-mediated RANKL-induced breast cancer cell migration by activation of the ERK and Akt pathway. Oncol Lett 2012;3:395-400. [PubMed]
- Liu Q, Russell MR, Shahriari K, et al. Interleukin-1 promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res 2013;73:3297-305. [PubMed]
- Baud V, Liu ZG, Bennett B, et al. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev 1999;13:1297-308. [PubMed]
- Xing L, Carlson L, Story B, et al. Expression of either NF-kappaB p50 or p52 in osteoclast precursors is required for IL-1-induced bone resorption. J Bone Miner Res 2003;18:260-9. [PubMed]
- Twillie DA, Eisenberger MA, Carducci MA, et al. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology 1995;45:542-9. [PubMed]
- Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1-20. [PubMed]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007;178:2623-9. [PubMed]
- Jee SH, Chu CY, Chiu HC, et al. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol 2004;123:1169-75. [PubMed]
- Zhao B, Li L, Wang L, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & Development 2012;26:54-68. [PubMed]
- Hall CL, Kang S, MacDougald OA, et al. Role of Wnts in prostate cancer bone metastases. J Cell Biochem 2006;97:661-72. [PubMed]
- Placencio VR, Sharif-Afshar AR, Li X, et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res 2008;68:4709-18. [PubMed]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781-810. [PubMed]
- Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 2004;25:1375-82. [PubMed]
- Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol 2005;23:2078-93. [PubMed]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117-29. [PubMed]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577-84. [PubMed]
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med 2005;353:172-87. [PubMed]
- Humphrey PA, Zhu X, Zarnegar R, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol 1995;147:386-96. [PubMed]
- Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002;60:1113-7. [PubMed]
- Boccaccio C, Andò M, Tamagnone L, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998;391:285-8. [PubMed]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298:1911-2. [PubMed]
- Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994;77:261-71. [PubMed]
- Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 2011;144:782-95. [PubMed]
- Pallares J, Rojo F, Iriarte J, et al. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol 2006;21:857-65. [PubMed]
- Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336-43. [PubMed]
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol 2001;280:C1375-86. [PubMed]
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem 1997;272:15442-51. [PubMed]
- Nagata K, Ohashi K, Nakano T, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 1996;271:30022-7. [PubMed]
- Goruppi S, Ruaro E, Varnum B, et al. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene 1999;18:4224-36. [PubMed]
- Hasanbasic I, Cuerquis J, Varnum B, et al. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 2004;287:H1207-13. [PubMed]
- Tai KY, Shieh YS, Lee CS, et al. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene 2008;27:4044-55. [PubMed]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34-45. [PubMed]
- Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 2006;12:1665-71. [PubMed]
- Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91-103;44.
- Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res 2009;69:2305-13. [PubMed]
- Dehm SM, Schmidt LJ, Heemers HV, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68:5469-77. [PubMed]
- Li Y, Chan SC, Brand LJ, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73:483-9. [PubMed]
- Liu P, Li S, Gan L, et al. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res 2008;68:10290-9. [PubMed]
- Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J Urol 2005;173:342-59. [PubMed]
- Russell PJ, Kingsley EA. Human prostate cancer cell lines. Methods Mol Med 2003;81:21-39. [PubMed]
- Navone NM, Olive M, Ozen M, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res 1997;3:2493-500. [PubMed]
- Navone NM, Rodriquez-Vargas MC, Benedict WF, et al. TabBO: a model reflecting common molecular features of androgen-independent prostate cancer. Clin Cancer Res 2000;6:1190-7. [PubMed]
- Ittmann M, Huang J, Radaelli E, et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013;73:2718-36. [PubMed]
- Valkenburg KC, Williams BO. Mouse models of prostate cancer. Prostate Cancer 2011;2011:895238.
- Goldstein AS, Witte ON. Does the microenvironment influence the cell types of origin for prostate cancer? Genes Dev 2013;27:1539-44. [PubMed]
- Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 2010;24:1967-2000. [PubMed]
- de Jong M, Maina T. Of mice and humans: are they the same?--Implications in cancer translational research. J Nucl Med 2010;51:501-4. [PubMed]
- Maser RS, Choudhury B, Campbell PJ, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 2007;447:966-71. [PubMed]
- Abate-Shen C, Shen MM. Mouse models of prostate carcinogenesis. Trends Genet 2002;18:S1-5. [PubMed]
- Powell WC, Cardiff RD, Cohen MB, et al. Mouse strains for prostate tumorigenesis based on genes altered in human prostate cancer. Curr Drug Targets 2003;4:263-79. [PubMed]
- Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 2003;3:952-9. [PubMed]
- Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002;100:3175-82. [PubMed]
- Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477-89. [PubMed]
- Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 2010;17:120-5. [PubMed]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332-7. [PubMed]
- Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891-906. [PubMed]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1:46-54. [PubMed]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 2005;5:675-88. [PubMed]
- Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis 2009;26:35-49. [PubMed]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol 2008;18:356-64. [PubMed]
- Kenny HA, Krausz T, Yamada SD, et al. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer 2007;121:1463-72. [PubMed]
- Li Q, Mullins SR, Sloane BF, et al. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 2008;10:314-29. [PubMed]
- Wang R, Xu J, Juliette L, et al. Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin Cancer Biol 2005;15:353-64. [PubMed]
- Hakkinen KM, Harunaga JS, Doyle AD, et al. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 2011;17:713-24. [PubMed]
- Chen CS, Mrksich M, Huang S, et al. Geometric control of cell life and death. Science 1997;276:1425-8. [PubMed]
- Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension. Science 2001;294:1708-12. [PubMed]
- Wolf K, Muller R, Borgmann S, et al. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 2003;102:3262-9. [PubMed]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673-87. [PubMed]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol 2002;4:E83-90. [PubMed]
- Hemler ME, Lobb RR. The leukocyte beta 1 integrins. Curr Opin Hematol 1995;2:61-7. [PubMed]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011;209:139-51. [PubMed]
- Patel VN, Knox SM, Likar KM, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 2007;134:4177-86. [PubMed]
- Yu X, Miyamoto S, Mekada E. Integrin alpha 2 beta 1-dependent EGF receptor activation at cell-cell contact sites. J Cell Sci 2000;113:2139-47. [PubMed]
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009;10:843-53. [PubMed]
- Gupta A, Cao W, Chellaiah MA. Integrin alphavbeta3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-kappaB ligand signaling axis. Mol Cancer 2012;11:66. [PubMed]
- Ziaee S, Chung LW. Induction of integrin alpha2 in a highly bone metastatic human prostate cancer cell line: roles of RANKL and AR under three-dimensional suspension culture. Mol Cancer 2014;13:208. [PubMed]
- Hall CL, Dubyk CW, Riesenberger TA, et al. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia 2008;10:797-803. [PubMed]
- Lamb LE, Zarif JC, Miranti CK. The androgen receptor induces integrin alpha6beta1 to promote prostate tumor cell survival via NF-kappaB and Bcl-xL Independently of PI3K signaling. Cancer Res 2011;71:2739-49. [PubMed]
- Edeleva EV, Shcherbata HR. Stress-induced ECM alteration modulates cellular microRNAs that feedback to readjust the extracellular environment and cell behavior. Front Genet 2013;4:305. [PubMed]
- Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res 2011;3:177-89. [PubMed]
- Lu P, Takai K, Weaver VM, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011;3:a005058. [PubMed]
- Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol 2001;33:33-44. [PubMed]
- Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997;137:231-45. [PubMed]
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol 2003;15:753-62. [PubMed]
- Chitcholtan K, Asselin E, Parent S, et al. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 2013;319:75-87. [PubMed]
- Lee GY, Kenny PA, Lee EH, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007;4:359-65. [PubMed]
- Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A 1998;95:14821-6. [PubMed]
- Windus LC, Kiss DL, Glover T, et al. In vivo biomarker expression patterns are preserved in 3D cultures of Prostate Cancer. Exp Cell Res 2012;318:2507-19. [PubMed]
- Miller BE, Miller FR, Heppner GH. Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res 1985;45:4200-5. [PubMed]
- Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159:176-87. [PubMed]


