Complete remission with tyrosine kinase inhibitors in renal cell carcinoma
In the era of cytokine therapy, although response rates of interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma (m-RCC) were not satisfactory, a small percentage of complete remission (CR) was achieved (1). Recently, targeted therapy has replaced cytokine therapy. Sunitinib, a tyrosine kinase inhibitor (TKI), has been especially effective in tumor reduction but has rarely induced CR (1). Because of the unique and characteristic adverse effects (AEs) of targeted drugs, this study highlights the importance of maintaining therapy in patients who have achieved CR with TKIs. A multicenter study on m-RCC patients who achieved CR with either TKI (sorafenib, sunitinib) alone or in combination with local treatment (surgery, radiotherapy, radiofrequency ablation) was performed retrospectively. The subjects of the study were 64 patients who achieved CR: 36 were treated with TKI alone and 28 with TKI plus local treatment. The denominator of all patients treated with TKIs was not available, but the incidence of CR from a part of them was 1.7%. This rate is in line with data from other studies (1,2). The aim of this study is to characterize the patients, to assess the indication of discontinuing targeted therapy, and to define the subsequent therapeutic implications.
Because of the small number of patients involved, this study could not definitively answer any one of the above questions. However, their findings do suggest some specific benefits.
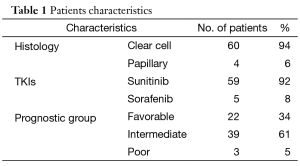
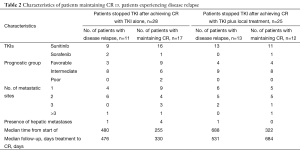
The patients’ characteristics are shown in Table 1. The majority of patients who attained CR were of favorable or intermediate risk, but 3 patients with poor risk also obtained CR. The fact that the majority of patients had received sunitinib was in line with the fact that sunitinib, with an approximately 30% reduction rate, has been shown to be more effective than sorafenib for tumor reduction (1). Table 2 compares the characteristics of patients who maintained CR with those who experienced disease relapse. None of the factors that predict a higher risk of relapse after discontinuation of TKIs was seen. In patients who achieved CR with TKI alone, the relapse rates varied: 44% for patients with TKI arrest at CR, 33% for patients with TKI arrest after further cycles of the same TKI, and 13% for those in which TKI administration was ongoing. Among them, there was no statistically significant difference. Similarly, in patients who achieved CR with TKI in combination with local treatment, the relapse rate for patients with TKI arrest at CR, for those with TKI arrest after further cycles of the same TKI, and for those with ongoing TKI administration were 52, 50, and 33%, respectively. Again, there was no statistically significant difference. These results may suggest a tendency for the relapse rate to decrease as the length of TKI therapy increases. Furthermore, in 14 of 26 patients, relapse occurred in a previously involved metastatic site. Considering the recurrence sites and the fact that all of the samples obtained by surgical resection included viable tumor-cells, (i.e. no patients achieved a pathological CR), it is suggested that tumors might arise where residual cancer cells have located.
Full table
Full table
On the other hand, although the overall response rate (ORR) of IL-2 treatment is approximately 15%, CR was attainable in up to 5% of m-RCC (1). Only 4 of 21 patients who achieved CR with IL-2 had a recurrence within four years (1). It is noted that IL-2 could induce such long term CR without additional administration. In this study, the median follow-up in maintaining the CR groups was short, 8-11 months; therefore the duration of CR cannot be discussed yet. However, CR obtained with targeted therapy seem to be essentially different from that obtained with immunotherapy. For example, targeted therapy directly inhibits tumor-growth signaling, whereas immunotherapy works indirectly via immune-cells, e.g., natural killer cells or cytotoxic T lymphocytes, and so on. As for achieving CR with immunotherapy, anti-cancer effects might be memorized by the immune-cells, and even after the cessation of treatment, those cells might continue to prevent disease recurrence.
On the other hand, as for achieving CR with targeted therapy, a rebound effect associated with the discontinuation of TKI might induce rapid re-growth and metastases by the residual cancer-cells (3). Moreover, the 1.7% of CR response rate for TKIs was evidently lower than that for IL-2 (1). Of the 64 patients, 28 had received TKI therapy and additional local treatment when they obtained the initial CR. Of the 26 patients, 11 patients received local treatment after recurrence. Furthermore, in this study, re-administration of TKIs did not induce any CR after recurrence.
Benefits arising from the discontinuation of therapy include a decrease in or absence of toxicity leading to the improvement of QOL, prevention of the development of resistance to drugs, and a reduction in the cost of treatment.
The fact that all patients in this study had previously undergone nephrectomy might have influenced the incidence of obtaining CR. Cytokine therapy significantly extended overall survival when nephrectomy was performed in the cytokine era (4). Therefore, we should always consider a multidisciplinary treatment, including surgery and radiotherapy, and not confine treatment to pharmacotherapy alone.
This study simply illustrates that TKIs can induce CR, either alone or in combination with local treatment. It also shows that CR was obtainable at every metastatic site and in every prognostic group. It does not clarify, however, whether or not discontinuation of therapy with TKIs after achieving CR is an acceptable strategy option. Further research is needed to determine that. Axitinib, a new TKI, is now in phase III of clinical trial for patients at high risk of recurrent renal cell carcinoma following nephrectomy as an adjuvant setting (5). In that trial, first a radical resection of tumor is firstly performed, similar to the CR status. Then axitinib is administrated to examine whether or not it reduces the rate of recurrence.
Although the trial situation differs from that of this study, the results of the adjuvant trial might answer some of the questions that this study could not due to the small number of eligible CR patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wada Y, Takahashi W, Kawano Y, et al. Current status of pharmacotherapy against metastatic renal cell carcinoma in Japan. Int J Urol 2012;19:284-95. [PubMed]
- Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 2009;10:757-63. [PubMed]
- Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006;116:2610-21. [PubMed]
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001;345:1655-9. [PubMed]
- SFJ Pharma To Conduct Phase III Trial Of Pfizer’s Axitinib Adjuvant Treatment In Asia. Asian Scientist Newsroom Tech & Pharma, 2012:1-2.


