Inflammation in prostate cancer progression and therapeutic targeting
Introduction
Prostate cancer remains one of the most treatable malignancies if caught early and actively observed. However, in late stage disease, tumors become androgen resistant and progress to lethal disease (1). Epidemiological data on the incidence of prostate cancer indicate that one out of every six men in the US will be diagnosed with prostate cancer in their lifetime (1). Approximately 28,000 American men, or 1 in every 33 diagnosed, lose their lives to prostate cancer each year, making it the 2nd leading cause of cancer-related death in American men (2). Prostate cancer advances through the accumulation of genetic and epigenetic changes that result in activation of oncogenes and the inactivation of tumor suppressor genes (3). Men from South East Asia experience much greater risk of prostate cancer in a single generation after immigration to the west, indicating the contribution of diverse epidemiological and environmental factors to prostate cancer risk (4). Prostate cancer proceeds from intraepithelial neoplasia to locally invasive, androgen-dependent neoplasia and eventually develops into androgen-independent metastatic disease [castration-resistant prostate cancer (CRPC)]. Poor survival outcomes in patients with advanced disease have focused intense effort toward development of therapeutic strategies for treatment of metastatic CRPC (5).
Growing evidence implicates chronic inflammation as a contributor to prostate cancer development and progression to advanced metastatic disease. A recent meta-analysis has established that prostatitis and sexually transmitted infections may be correlated with increased prostate cancer risk (6). Intake of anti-inflammatory drugs and antioxidants has been associated with decreased prostate cancer risk. Additionally, genetic experimentation has identified RNASEL, encoding an interferon inducible ribonuclease, and macrophage scavenger receptor 1 (MSR1), encoding subunits of the MSR, as inherited susceptibility genes for familial prostate cancer (7). Somatic silencing of GSTP1, encoding a glutathione S-transferase capable of defending against oxidant damage, is detected in almost all prostate cancer cases (8). Proliferative inflammatory atrophy (PIA), containing activated inflammatory cells and proliferating epithelial cells, has emerged as a pathological precursor to prostatic intraepithelial neoplasia (PIN) lesions and prostatic cancer (7).
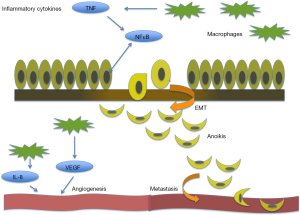
Inflammatory gene markers are associated with a poor prognosis in many cancers including breast cancer (9). Chronic inflammation is thought to have a significant effect on prostate cancer progression and metastasis through angiogenesis and epithelial mesenchymal transition (EMT), impacting the dynamics of the tumor microenvironment (Figure 1). Progression of CRPC to metastatic disease unresponsive to anti-androgen therapy (5), calls for a reliable marker to predict aggressive clinical presentation and navigate therapeutic targeting for individual patients. When defining the tumor profile of prostate cancer patients and tailoring individual treatment strategies, the significance of inflammation in the context of its functional impact in the tumor microenvironment must be considered. This review focuses on the current translational evidence linking the process of inflammation with prostate cancer development, progression and therapeutic response.
The tumor microenvironment dictating cell fate
The extracellular matrix (ECM) is a critical regulator of cell proliferation, migration, differentiation, and ultimately cell survival in normal and tumorigenic growth (9). The ECM is a complex network of laminin, collagen, fibronectin and proteoglycans, providing structural and biochemical support to surrounding cells, and is active in regulating cell communication, proliferation, and survival. In healthy cells, disruption to the ECM leads to caspase-dependent apoptosis. However, in the setting of chronic inflammation, a different process is observed. Notably, a wide number of cytokines are produced by inflammatory cells, such as tumor necrosis factor (TNF), interleukin-7 (IL-7), interleukin-2 (IL-2), RANTES, and macrophage inflammatory protein-1b. In addition, the chronic inflammatory cascade activates growth factors including basic fibroblast growth factor (bFGF) and transforming growth factor-β (TGF-β). The release of these soluble inflammatory mediators into the ECM activates surrounding stromal cells (10) and the formation of the reactive stroma dramatically rearranges the landscape of ECM (11). This reactive stroma, contributes to a cytokine-rich inflammation-conditioned microenvironment that nurtures tumor cells towards prostate cancer metastasis illustrating the dynamic nature of the ECM (12). Consequential to inflammation-mediated disruption of ECM, resulting mesenchymal prostate cells that are resistant to anoikis acquire invasive and migration properties towards aggressive tumor growth and metastasis (Figure 1).
Metastasis is a highly complex process that involves dissociation from the original organ, detachment from the ECM, migration of the cell, resistance to anoikis, invasion of surrounding tissue, cell adhesion, colonization, and eventually cancer growth in a distant organ. Microarray analysis identified marked changes in ECM composition during tumor growth, and these changes are detected through the quantitative shift in the expression of specific proteins within the ECM (9). Cancer is primarily lethal because of its ability to metastasize (9). The majority of deaths from carcinoma are caused by secondary growths that arise from metastasis (13). Death from prostate cancer is caused by metastasis to the retroperitoneal and pelvic lymph nodes, as well as to the bone (1).
Normally, cells that detach from the ECM are unable to survive and proliferate, and subsequently die via the phenomenon of anoikis. Anoikis, Greek for “homeless”, is a specialized mode of cell death that occurs due to a lack of extracellular connections to the ECM and adjacent cells. In tumor cells, resistance to anoikis confers a survival advantage enabling the cell to travel and reattach through the process of intravasation (14). Apoptosis regulators, such as Bcl-2, p53, and FLICE inhibitory protein, coordinate the functional overlap or “bypassing” relationship of apoptosis with anoikis and autophagy (5) that may impact cell-cell communication via focal adhesion complexes (15). Apoptosis has two main pathways, intrinsic (mitochondria dependent) or extrinsic (death receptor dependent) (16). The intrinsic pathway is typically activated by cellular stressors, such as low oxygen levels, infection, and UV damage. Extrinsic apoptosis, by TNF, has a close functional relationship with inflammation and cancer.
Angiogenesis, the formation of new blood vessels, is associated with many inflammatory diseases, including psoriasis and rheumatoid arthritis, but the relationship is typically controlled (17). Cells chronically activated by the inflammatory cascade release cytokines that induce vascularization via an intense angiogenic response. Tumor cells can promote angiogenesis by releasing a number of growth factors, including vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), and bFGF, supporting capillary growth and increasing vascularity. In normal healthy cells, VEGF promotes wound healing and plays a critical role in creating new vessels to bypass blocked vessels. In cancer, overexpression of VEGF is associated with excessive tumor vascularity, giving rise to aggressive tumors, and ultimately resulting in poor patient prognosis.
Impact of inflammation on prostate cancer initiation and progression
Intraprostatic inflammation is frequently detected in prostate biopsies in patients with elevated prostate specific antigen (PSA) (18). Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer (19). Significantly enough an inflammatory effector Pentraxin 3 has recently been identified as a biomarker for predicting tumor progression due to prostatic inflammation in prostate cancer patients (20).
PIN is characterized by irregular epithelial cells that develop around inflammatory cells. The relationship of PIN lesions and prostate cancer may provide strong evidence for the direct relationship of prostate cancer and inflammation. When PIN lesions are found in biopsies, the chance of finding a neoplasm jumps to 30% (21). High-grade PIN lesions are found in 85-100% of radical prostatectomy patients (22). Despite this high prevalence, the impact of prostatic inflammation on prostate cancer progression to advanced metastatic disease has been challenging to delineate. First, it is difficult to calculate the incidence of prostatitis because the condition is often asymptomatic. As many as 5-10% of men over 40 have diagnosed prostatitis, but many more may have the disease and do not display symptoms. Asymptomatic prostatitis is often identified within prostate biopsies, but is not diagnosed prior to the development of the neoplasm (23). Secondly, men with symptoms of prostatitis are more likely to be diagnosed with prostate cancer as a result of increased prevalence of biopsy (24). While the increasing number of biopsies performed for this reason may result in biased reporting of prostate cancer incidence, one must consider that prostate adenocarcinoma may exist in men without a history of prostatitis, but may remain undetected due lack of symptoms and slow growth kinetics of the tumor.
Development of prostatitis does not have a consistent genetic footprint. The clinical condition may be caused by a variety factors including pathogens, chemical and physical trauma, diet, or a combination of these factors. Urine reflux, or the abnormal flow of urine from the bladder back through the ureters, is thought to cause chemical irritation that may cause chronic inflammation in the prostate (25). Non-sexually transmitted organisms such as E. coli and Propionibacterium acnes are shown to cause acute and chronic prostatitis (26,27). Presence of many sexual transmitted organisms, including Neisseria gonorrhea (28) and Chlamydia trachomatis (29), characterized by chronic infection and inflammation, coincides with a potential increase in prostate cancer risk. An increased risk of prostate cancer in men with a history of inflammatory sexually transmitted diseases (STDs) provides an indirect link between chronic inflammation in prostate tumorigenesis (30). Considering the variability and diversity in the causative factors for prostatitis, it might be argued in favor of inflammation being the ubiquitous factor associated with increased incidence of prostate cancer, without an association with any specific pathogen or environmental factor. Inflammation as a biological process is typically associated with infection, but it can be triggered by autoimmune diseases, allergies, and tissue trauma. Cytokines released by inflammatory cells activate signal cascades providing a growth advantage for surrounding tumors. Many cytokine functions and selectin-ligand interactions can be manipulated to promote growth (31). Inflammatory cells contribute to increased vascularity, DNA damage, cytoskeleton remodeling and ECM degradation to provide a nurturing growth microenvironment (32). Chronic inflammation of the prostate can be caused by constant infection, which is known to promote DNA damage due to persistent production of oxygen and nitrogen species that are produced by leukocytes to fight infection (33). Mutations in p53 tumor suppressor are often found in chronically inflamed cancer cells (34). An intrinsic pathway linking inflammation to cancer involves such genetic changes, while an extrinsic pathway proceeds via microbial infections causing chronic inflammation via activation of inflammatory mediators (35). Rapidly growing evidence links inflammation to the initiation and progression of many human cancers to advanced metastatic disease including colon, lung and prostate cancer (36).
The patient’s response to the cytokines released in the inflamed cells may have a potentially significant effect on prostate cancer initiation. Specifically, two genes can contribute to prostate cancer susceptibility. RNASEL, a ribonuclease that degrades viral RNA and the MSR1 influence the host response to infection (37). RNASEL, is a prostate cancer susceptibility gene, with RNASEL alleles GLU256X and Met1Ile both correlating with prostate cancer among family lines. RNASEL allele Arg462Gln was also shown to have an increased prostate cancer risk in a population controlled study (38). These alleles encode defective enzymes that increase the risk of inflammatory related prostate cancer (37). MSR1 defects are also linked to increased prostate cancer risk in family lines. ARG293x mutation of MSR1 was found in 2.52% of men with sporadic prostate cancer but only 0.39% without prostate cancer (39). Recent epidemiological evidence however built on the Prostate Cancer Prevention Trial (PCPT) database and outcomes, provides the first etiologic link between chronic inflammation and prostate progression to aggressive disease, suggesting a platform for prevention of prostate cancer metastasis by targeting prostatic inflammation (19).
TNF is a physiologic cytokine that is a prominent mediator of both acute and chronic inflammation (40). TNF is itself a cytokine, but also stimulates the release of many additional cytokines and chemokines involved in pathologic processes of autoimmune inflammatory reactions (40). Recent experimental data has proven TNF to be a critical mediator of cancer-associated chronic inflammation, which may promote tumor growth and spread (40,41). These inflammatory conditions trigger immune and stromal cell release of soluble mediators, which facilitate the progression of cancerous lesions (42).
Nuclear Factor Kappa B (NFκB) is a member of a family of transcription factors that are activated in many types of cancer cells. NFκB transcription factors are activated by numerous converging signaling cascades, primarily the pro-inflammatory cytokine, TNF (43). Once activated, this family of transcriptions factors is involved in regulating gene expression driving cell growth, angiogenesis, and metastasis (43). Prostate cancer cells have been found to express high levels of NFκB (43). NFκB may promote cell growth and proliferation in prostate cancer cells by regulating the expression of cell cycle controlling genes, including c-myc, cyclin D1, and IL-6 (44). Furthermore, the expression of angiogenic factors, VEGF and IL-8, is regulated by the activation of NFκB transcription factors (44). Constitutive NFκB activity within prostate cancer cells has been suggested to have prognostic indications for prostate cancer tumors (43).
The phenotypic process of EMT was first identified to be critically required for organogenesis during development (9). In normal and malignant epithelial cells, physiologic activation of the EMT program depends on the convergence of multiple molecular signals from the microenvironment. TGF-β, an inflammatory cytokine released in the setting of reactive stroma, induces the process of EMT. In carcinoma cells, mesenchymal traits acquired during activation of the EMT program set in motion the steps of an invasion-metastatic cascade sequence (45). This signal sequence stimulates carcinoma cells to invade in the locality of the primary tumor, intravasate, travel through circulation, extravasate, survive in the parenchyma of distant organs, seeding microtumors which may eventually form true metastatic macrotumors (45). Epithelial cells signaled by EMT programming gain dedifferentiated stem-cell like properties, resulting in cancer stem cells (CSCs) (45). In the setting of neoplasm, these stem-like properties impart tumor-initiating characteristics that are crucial for future metastasis. High-grade tumors associated with poor patient prognosis often express molecular signatures of the EMT landscape. One must consider that mesenchymal attributes gained by epithelial cells in response to various physiological stimuli are not phenotypically permanent and this is the very essence of the transient nature of the phenomenon. The EMT programing does not result in complete epithelial dedifferentiation to pure mesenchymal properties, rather cells expresses a spectrum of epithelial and mesenchymal attributes (45). The plasticity of the EMT signal cascade suggests the importance of epigenetic regulators in gene expression (45). Activation of EMT by TGF-β causes the cell to change both its surroundings and its structural relationship with the ECM landscape and the tumor microenvironment (46). Loss of E-cadherin is hall marker event in EMT and the metastasis of many cancers, including prostate cancer that confers increased cell mobility, decrease in polarity and enhanced invasion due to loss of adherent junctions, allowing for cells to free from interactions with neighboring cells (47) and upon surviving anoikis to rapidly move. Transcriptional repressors such as Snail, Twist, Par6 and NFκB have been identified as coordinating EMT by inhibiting E-Cadherin expression in aggressive tumors (48).
High levels of tumor associated macrophages (TAM) are found in tumors (49,50). Although many different cell types interact within a tumor, almost 50% of tumor mass is made of macrophages (51). TAMs, present in large numbers in tumor tissues, are key promoters of cancer-related inflammation (52). Circulating monocytes are recruited to tumors by various chemokines and cytokines including CCL2 and M-CSF (52). Once in the tumor microenvironment, monocytes are transformed into TAMs that promote and maintain chronic inflammation (52). Initially, TAMs may harbor some tumor cell-killing action by cytokine release (52). Paradoxically, continued release of cytokines, growth factor, and ECM (IL-6, VEGF) protein by TAMs result in tumor cell proliferation, angiogenesis, and metastatic spread (52). A close causal relationship has been established by in vivo studies in models of tumorigenesis such as in the MMTV-PyMT mice, which spontaneously develop breast cancer tumors; upon crossing with mice that lack monocytes and macrophages, there was a significant decrease in metastasis. In a similar study, PyMt mice were crossed with mice lacking colony stimulating factor-1 (CSF-1) during early tumor development due to a null mutation in the CSF-1 gene (51). Mice lacking CSF-1 had the same rate tumor cell proliferation, while they exhibited a distinctly lower recruitment of macrophages to the tumor site. Targeting the CSF-1 null gene restored macrophage recruitment, which in turn increased metastatic potential in the mice. One cannot however dismiss the complex relationship between TAM and tumor growth as both positive and negative effects on tumor growth, are associated with TAM action (51).
Targeting inflammation-related cancer
There is rapidly growing evidence for the impact of nonsteriodal anti-inflammatory drugs (NSAIDs) on cancer (53). In colon cancer NSAIDs are known to reduce cancer risk by 40-50% (54). At the cellular level, NSAIDs target cyclooxygenase (COX)-2, which is expressed in inflammatory cells, specifically in the prostate and in PIA widely considered to be a precursor of prostate cancer (7,19). Multiple experimental studies have documented that COX-2 overexpression in prostate cancer can be effectively targeted by COX-2 selective inhibitors such as celecoxib (55). A recent meta-analysis evaluated the efficacy of NSAIDs in reducing prostate cancer risk, based on twenty observation studies with a total of 25,768 participates included. The clinical data available indicate that NSAIDs had a 5-8% protective effect against prostate cancer (55). When NSAIDs and aspirin (ASA) were analyzed independently there was a statistically significant protective effect as revealed by the risk reduction at 5% for ASA and 8% for other NSAIDs (55). A population based case-control study of 1,900 men analyzed the use of ASA to reduce prostate cancer risk (56). Men who reported use of ASA had a statistically significant 18% reduction of relative risk of prostate cancer (56). Men who currently used ASA had a statistically significant 21% reduction in relative risk (56). Long-term users of ASA had a statistically significant 24% reduction in relative risk (56). Men who took daily low dose ASA had a 29% risk reduction, compared to men not taking (56). Use of daily low dose ASA was associated with the highest risk reduction, showing the strongest inverse relationship between ASA use and prostate cancer (56). Growing epidemiological and experimental evidence has documented an inverse relationship between ASA use and prostate cancer, with a statistical significance being reached after 5 or more years of use in men with metastatic disease (57). Moreover, in a large multicenter study of over 90,000 men in the Kaiser Permanente Medical Care program, a direct protective effect was observed in ingesting six ASAs daily (58). In addition, an independent study revealed that NSAIDs other than ASA, were only effective for older patients (over age of 60) (59). Compelling experimental evidence has significantly implicated the potential benefit of NSAID and ASA use for the prevention of prostate cancer in the setting of chronic inflammation. The challenge however has been to specifically design the dosing and/or schedule that would confer the highest benefit.
Owing to the critical role of pro-inflammatory cytokine TNF and transcription factor NFκB in regulating cell cycle growth, angiogenesis, and cancer metastasis, these signaling molecules emerge as powerful therapeutic targets. Mechanistic blockade of the upstream signal cascade for NFκB activation can be achieved using proteasome inhibitors, IκB kinase (IKK) inhibitors, and antioxidant molecules (44). Proteasome inhibitors have been shown to have antitumor activity in multiple types of cancers, including prostate cancer (44). Recently a new proteasome inhibitor, bortezomib, was found to inhibit the 26s proteasome, which is the principle regulator of intracellular protein regulation (44). This drug inhibits the degradation of protein molecules involved in cell cycle regulation, currently in clinical use in multiple myeloma patients.
Several NSAIDs including ASA and COX-2 inhibitors have been shown to suppress activation of NFκB by suppressing IKK (44). Recent in vitro evidence identified the ability of COX-2 inhibitor, Celecoxib, to suppress NFκB activation by TNF via suppressing IKK (44). Sulfasalazine and other anti-inflammatory molecules are being tested for the potential ability to inhibit activation of NFκB by suppressing IKK (44). Resveratrol is also proven to be a potent NFκB inhibitor through its ability to suppress IKK activity (44) and it is currently being evaluated in clinical studies as a potential anticancer therapeutic agent. The second method of inhibiting NFκB enforces targeting of the transcription factor directly (44). Elegant studies have attempted molecular approaches specifically blocking the DNA binding of NFκB, inhibiting the activation of NFκB using glucocorticoids, as well as functionally interfering with the downstream mRNA products of NFκB (44). Recent investigative efforts have utilized oligodeoxynucleotides (ODN) as decoys to block the binding of NFκB transcription factors to DNA promoter regions for target genes (44). Directly injecting decoy ODN into tumors leads to downregulation of downstream NFκB cell cycle gene products. Moreover ODN decoys have been shown to overcome therapeutic resistance in gastric cancers in response to treatment with chemotherapeutic 5-FU (44). Therapeutic agents impairing activation of transcription factor NFκB signaling can be potentially used as adjuvant therapies in combination with other current treatment modalities, since several signaling cascades can activate NFκB, including TNF (40). Therefore, it is unlikely that a single agent would have the potential to inhibit NFκB, however its critical role in cell cycle regulation, apoptosis, angiogenesis and metastasis, heavily supports therapeutic promise in targeting NFκB as an anticancer agent (44).
High-grade prostate tumors associated with poor patient prognosis often contain cells that express molecular signatures consistent with the EMT program (45). Dedifferentiated CSCs cells expressing mesenchymal attributes promote tumor growth and metastasis (45). Identification of epigenetic molecular signaling pathways that drive the EMT program can provide new therapeutic targets to potentially prevent the epithelial to mesenchymal transformation. Several experimental efforts have focused on targeting epigenetic regulators. The DNA demethylating agent 5-azacytidine can restore expression of epithelial microRNA (45). Similarly, histone deacetylase (HDAC) inhibitors have been shown to stimulate E-cadherin expression (45). Ongoing efforts pursue the impact of chromatin-modifying enzymes on driving EMT (45), via regulating epigenetic modifications conferring the EMT characteristics in cancer epithelial cells during progression to metastasis.
Several pre-clinical and clinical studies have interrogated the association between high TAM density with poor patient prognosis and therapeutic resistance (52). TAMs are considered attractive targets for antitumor intervention (52) since monocyte/TAM depletion can markedly impair tumor growth and metastatic spread. Moreover depleting TAMs from the tumor microenvironment has led to better response to conventional chemotherapy and antiangiogenic agents (52). Trabectedin (ET-743) is a new agent found to be selectively cytotoxic in vitro to human monocytes, in addition to inhibiting the production of some cytokines (CCL2, IL-6) (52). Recent studies investigated the in vivo effects of trabectedin in tumor-bearing mice (52), in three transplantable tumor models, the MN/MCA1 fibrosarcoma, the ID8 ovarian carcinoma and Lewis lung carcinoma. Analysis of the antitumor activity of trabectedin on these tumor models revealed that this agent significantly delayed tumor growth, including decreased lung metastasis from the MN/MCA1 tumor line (52). Flow cytometric analysis revealed that the percentage of TAMs was significantly lower in all tumor models (mean inhibition: MN/MCA1, 38%; LLC, 46%; ID8, 43%) (52). These studies also demonstrated that TAM population was reduced by more than 50%, and the mRNA levels for CCL2 were significantly lower after treatment with trabectedin (52). The clinical relevance of these experimental findings, were validated by clinical studies investigating the impact of trabectedin on soft tissue sarcoma patients receiving trabectedin as a single treatment. Immunohistochemical profiling established that trabectadin treatment resulted in a decrease in the density of TAMs in soft tissue sarcoma (52). Moreover patients receiving therapy had a significant monocyte reduction post-treatment from 9.4% to 3.2% (52). These results further support the emerging value of macrophage targeted antitumor therapies, tailored to a personalized-medicine approach (52).
Conclusions
Chronic inflammation has been proven as an integral contributor to cancer ontogeny. Dissecting the relationship between prostate cancer evolution and the process of inflammation is an ongoing pursuit. Current observation and experimentation clearly demonstrate that inflammation is functionally synergistic with high-grade aggressive prostate tumors and ultimately metastatic spread (7,19). The evidence-based knowledge so far supports the regulatory role of the inflammatory cascade in prostate cancer growth and progression to metastasis by exerting a tight control on the tumor microenvironment landscape, the ECM and EMT cycling outcomes. Inflammatory cells within the tumor microenvironment promote angiogenesis by releasing a number of growth factors, including VEGF, IL-8, and bFGF during tumorigenic growth (Figure 1). The release of soluble inflammatory mediators such as TNF-α, TGF-β, IL-7, IL-2, RANTES, and macrophage inflammatory protein-1b activates surrounding stromal cells and provokes dramatic remodeling of the ECM (10). Further inflammatory cytokines released in the setting of reactive stroma, actuate the cellular response towards induction of EMT. Indeed, the multiplicity of signaling factors influencing inflammation in the prostate microenvironment and the complexity of biological events linking inflammation to prostate cancer progression and metastasis provide a promising platform for potential therapeutic targeting. To date, numerous investigative efforts have exploited the anti-tumor efficacy of non-steroidal anti-inflammatory agents, modulators of inflammatory cytokine and transcription factor activation, epigenetic modification of the EMT phenotypic program dictating cell polarity and invasiveness and repression of TAM signaling.
Acknowledgements
These studies were supported by the James F. Hardymon Endowment for Urology Research at the University of Kentucky and an NIH (National Institutes of Health) grant DK 083761.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med 2004;351:1488-90. [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol 2004;171:S36-40. [PubMed]
- Peto J. Cancer epidemiology in the last century and the next decade. Nature 2001;411:390-5. [PubMed]
- Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res 2005;65:11230-5. [PubMed]
- Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology 2002;60:78-83. [PubMed]
- De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007;7:256-69. [PubMed]
- Nelson WG, De Marzo AM, DeWeese TL, et al. The role of inflammation in the pathogenesis of prostate cancer. J Urol 2004;172:S6-11; discussion S11-2.
- Horbinski C, Mojesky C, Kyprianou N. Live free or die: tales of homeless (cells) in cancer. Am J Pathol 2010;177:1044-52. [PubMed]
- Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013;218:1402-10. [PubMed]
- Cunha GR, Hayward SW, Wang YZ, et al. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003;107:1-10. [PubMed]
- Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer 2012;19:R187-204. [PubMed]
- Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev 2012;31:285-93. [PubMed]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001;13:555-62. [PubMed]
- Sakamoto S, McCann RO, Dhir R, et al. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res 2010;70:1885-95. [PubMed]
- Hotchkiss RS, Strasser A, McDunn JE, et al. Cell death. N Engl J Med 2009;361:1570-83. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Schatteman PH, Hoekx L, Wyndaele JJ, et al. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol 2000;37:404-12. [PubMed]
- Gurel B, Lucia MS, Thompson IM Jr, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 2014;23:847-56. [PubMed]
- Stallone G, Cormio L, Netti GS, et al. Pentraxin 3: a novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Cancer Res 2014;74:4230-8. [PubMed]
- Leite KR, Camara-Lopes LH, Cury J, et al. Prostate cancer detection at rebiopsy after an initial benign diagnosis: results using sextant extended prostate biopsy. Clinics (Sao Paulo) 2008;63:339-42. [PubMed]
- Godoy G, Taneja SS. Contemporary clinical management of isolated high-grade prostatic intraepithelial neoplasia. Prostate Cancer Prostatic Dis 2008;11:20-31. [PubMed]
- Roberts RO, Lieber MM, Rhodes T, et al. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology 1998;51:578-84. [PubMed]
- Krieger JN, Riley DE, Cheah PY, et al. Epidemiology of prostatitis: new evidence for a world-wide problem. World J Urol 2003;21:70-4. [PubMed]
- Kirby RS, Lowe D, Bultitude MI, et al. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol 1982;54:729-31. [PubMed]
- Shinohara DB, Vaghasia AM, Yu SH, et al. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate 2013;73:1007-15. [PubMed]
- Elkahwaji JE, Zhong W, Hopkins WJ, et al. Chronic bacterial infection and inflammation incite reactive hyperplasia in a mouse model of chronic prostatitis. Prostate 2007;67:14-21. [PubMed]
- Pelouze PS. eds. Gonorrhea in the male and female: a book for practitioners. Philadelphia: W. B. Saunders Company, 1935.
- Poletti F, Medici MC, Alinovi A, et al. Isolation of Chlamydia trachomatis from the prostatic cells in patients affected by nonacute abacterial prostatitis. J Urol 1985;134:691-3. [PubMed]
- Hayes RB, Pottern LM, Strickler H, et al. Sexual behaviour, STDs and risks for prostate cancer. Br J Cancer 2000;82:718-25. [PubMed]
- Koong AC, Denko NC, Hudson KM, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res 2000;60:883-7. [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [PubMed]
- Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc) 1998;63:854-65. [PubMed]
- Yamanishi Y, Boyle DL, Rosengren S, et al. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A 2002;99:10025-30. [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [PubMed]
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med 2000;248:171-83. [PubMed]
- Goldstraw MA, Fitzpatrick JM, Kirby RS. What is the role of inflammation in the pathogenesis of prostate cancer? BJU Int 2007;99:966-8. [PubMed]
- Casey G, Neville PJ, Plummer SJ, et al. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet 2002;32:581-3. [PubMed]
- Zheng SL, Liu W, Wiklund F, et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate 2006;66:1556-64. [PubMed]
- Chu WM. Tumor necrosis factor. Cancer Lett 2013;328:222-5. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [PubMed]
- Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer - more than a "gut" feeling? Cell Div 2010;5:14. [PubMed]
- Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem 2004;91:100-17. [PubMed]
- Lee CH, Jeon YT, Kim SH, et al. NF-kappaB as a potential molecular target for cancer therapy. Biofactors 2007;29:19-35. [PubMed]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013;19:1438-49. [PubMed]
- Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res 2012;347:85-101. [PubMed]
- Gravdal K, Halvorsen OJ, Haukaas SA, et al. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res 2007;13:7003-11. [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-6. [PubMed]
- Pu H, Collazo J, Jones E, et al. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res 2009;69:7366-74. [PubMed]
- Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009;86:1065-73. [PubMed]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71-8. [PubMed]
- Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249-62. [PubMed]
- Bowers LW, Maximo IX, Brenner AJ, et al. NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions. Cancer Res 2014;74:4446-57. [PubMed]
- García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology 2001;12:88-93. [PubMed]
- Jafari S, Etminan M, Afshar K. Nonsteroidal anti-inflammatory drugs and prostate cancer: a systematic review of the literature and meta-analysis. Can Urol Assoc J 2009;3:323-330. [PubMed]
- Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol 2010;172:578-90. [PubMed]
- Leitzmann MF, Stampfer MJ, Ma J, et al. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2002;11:1108-11. [PubMed]
- Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control 2002;13:427-34. [PubMed]
- Roberts RO, Jacobson DJ, Girman CJ, et al. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc 2002;77:219-25. [PubMed]


