Outlining the limits of partial nephrectomy
Introduction
Partial nephrectomy (PN) has evolved to become standard of care for small renal masses (SRMs) and select larger tumours that are amenable to nephron-sparing techniques. Early studies of cT1a (≤4 cm) tumours demonstrated that PN compared to radical nephrectomy (RN) was associated with improved overall survival (OS) (1-3). It is nevertheless important to acknowledge that the European Organization for Research and Treatment of Cancer Genito-Urinary (EORTC-GU) noninferiority phase 3 trial 30904 demonstrated improved OS for RN; however, in the renal cell carcinoma (RCC) subgroup, this trend lost significance (4). However, the increased recognition that chronic kidney disease (CKD) significantly impacts medical morbidity (5-8) has led the American Urological Association (AUA) and European Association of Urology (EAU) guidelines to support PN as the procedure of choice for cT1a tumours (9,10).
Laparoscopic PN (LPN) and robot-assisted PN (RAPN) as minimally-invasive alternatives to the traditional open PN (OPN) have seen increased utilization. While these techniques are associated with reduced postoperative pain and shorter lengths of stay all the while maintaining comparable oncologic outcomes to OPN (11,12), the scope of this review is not focused on the minimally invasive limits of PN. In this review, we aim to analyze tumour staging, renal functional, anatomical, and surgical factors to define limitations where PN may represent significant oncological risk or surgical morbidity, tipping the balance in favour of RN.
Tumour staging considerations
Primary tumour size
Nearly 25% of patients with RCC present with underlying CKD (13) and thus also carry a higher risk of cardiovascular comorbidities (14,15). This has led to increased consideration of PN for larger, specifically T2, renal masses (16). The oncological benefit in this setting remains controversial. Compared to RN, studies have reported equivalent recurrence-free, cancer-specific as well as OS rates for PN in lesions 4-7 cm in size (17-21). The boundaries of what is considered feasible for PN have expanded to also include more complex tumour locations (17). An analysis of the Surveillance, Epidemiology, and End Results (SEER) database from 1998 to 2008 identified no statistically significant difference in 5-year cancer-specific mortality between RN and PN for lesions T2 or greater (P=0.2) (22). Given that tumour complexity may also influence PN selection, Kopp et al. utilized the radius, endophyticity, nearness to collecting system, anterior/posterior, and location polarity (RENAL) nephrometry score to compare T2 masses treated by either RN (n=122) or PN (n=80) (23). After a median follow-up of 41.5 months, no significant differences were identified between median RENAL score and 5-year progression-free survival (69.8% vs. 79.9% for RN and PN, respectively; P=0.115) or cancer-specific survival (82.5% vs. 86.7% for RN and PN, respectively; P=0.407).
The Mayo Clinic’s experience of PN for T2, T3a, and T3b lesions was assessed by matching to a reference RN cohort by stage, tumor size, baseline renal function, age, and gender (24). PN, when compared to RN, was not associated with an increased risk of death from all causes (HR 1.11, 95% CI, 0.72-1.71; P=0.642) or RCC-specific mortality (HR 0.80, 95% CI, 0.43-1.50; P=0.489). Further, after a median follow-up of 3.2 years, 15 PN patients (22%) and 69 RN patients (33%) had metastatic disease (HR 0.74, 95% CI, 0.42-1.29; P=0.234). More recently, outcomes of PN on masses ≥7 cm (including 41% >10 cm) were reported by Long et al. In this series of 46 patients, the 5- and 10-year overall and RCC-specific survival rates were 94.5% and 70.9%, respectively (25).
Locally advanced tumours
Invasion and development of a thrombus of the renal vein or inferior vena cava (IVC) associated with a renal mass has historically been managed with RN and thrombectomy (26). Utilization of a nephron sparing surgery (NSS) in this situation remains controversial. It is motivated by preserving renal function in patients expected to have adequate life expectancy, given that the 5-year cancer specific survival is 40-65% in patients with locally advanced RCC, particularly with favorable prognostic factors (27). PN may have a role in this setting; however, the data to support this is limited. In one study comparing the oncologic outcomes based on the surgical technique in T2-T3b tumours, 34 patients underwent PN and 567 patients received RN (28). Disease recurrence was observed in four of the 34 PN patients (12%) versus 164 of the 567 (28.9%) in the RN cohort at a median follow up of 24.2 and 13.2 months, respectively. While this may reflect a significant selection bias, wherein patients receiving PN likely also had more favorable comorbidities, on multivariate analysis, the type of surgical procedure was not an independent predictor of disease recurrence or RCC-specific death. It should be noted that there were tradeoffs in performing PN—namely, a higher procedure-related complication rate in three patients (9%): two had a prolonged urinary fistula, successfully managed with ureteric stenting, and one patient had hemorrhage requiring emergent re-exploration.
Cytoreductive surgery for metastatic RCC
Approximately 17-30% of patients with RCC present with metastatic disease (29). In appropriately selected patients, cytoreductive nephrectomy remains an important consideration, even in the contemporary tyrosine kinase inhibitor era (30). However, in light of the even shorter life expectancy of patients with metastatic RCC than those with locally advanced cancer, the relative benefit of nephron preservation has to be appropriately balanced with the risk of peri-operative morbidity to select candidates with favorable prognosis.
There are limited reports demonstrating the utility of PN in the metastatic setting. Krambeck and colleagues compared 16 patients who underwent cytoreductive PN and compared their results to 404 patients who underwent RN for cytoreduction (31). Of the 16 patients, 12 had a solitary kidney, which is an important imperative consideration with significant quality of life implications. The cancer specific survival rates of these 16 patients at 1, 3, and 5 years were 81%, 49%, and 49%, respectively. The cancer specific survival rates of the 404 patients who underwent RN at 1, 3, and 5 years were 51%, 21%, and 13%, respectively.
The feasibility and prevalence of cytoreductive PN was assessed by Capitanio et al. using the SEER cancer registry from 1988 to 2004 to identify 46 patients that received cyroreductive PN. This cohort was compared to a historical control group from 1997 that underwent RN (32). Multivariate analysis demonstrated no statistically significant difference in cancer specific survival between the two groups (HR 1.40; P=0.16). Additionally, Hellanthal and colleagues analyzed the SEER database from 1988 to 2005 and identified 70 patients with metastatic disease that underwent PN (2%). These patients were 0.49 times less likely to die from RCC than those who underwent RN (P<0.001) (33).
Babaian and colleagues examined the MD Anderson Cancer Center’s experience with metastatic RCC patients who underwent PN from 1996 to 2011 (29). Of the 33 patients, 22 patients (67%) died from disease at a median follow up of 27 months. Patients that received PN for either a metachronous contralateral renal mass or a renal mass <4 cm had the best OS (61 and 42 months, respectively).
Renal function considerations
The main advantage of PN over RN is nephron preservation, leading to improved postoperative renal function. However, PN is still associated with some functional decline as the procedure inherently excises nephrons adjacent to the tumor and eventual reconstruction is required, which can lead to devascularization. Renal function after PN depends on the three “Qs”: quality [baseline glomerular filtration rate (GFR)], quantity (percentage of renal function preserved), and quickness (ischemia time) (34). Many studies have demonstrated the importance of the “quality” factor, viewing the baseline GFR as the determinant of ultimate renal function following PN (35,36). Effective PN focuses on improving the precise excision of the tumor with minimal margins with careful reconstruction to maximize the number of preserved nephrons all the while minimizing the amount of ischemic injury associated with the procedure. The duration of ischemia remains an important surgeon-modifiable factor (37) and novel techniques to reduce it have shown promise (38).
To our knowledge, apart from end stage renal disease, there is no reliable lower-limit GFR threshold beyond which PN should not be attempted. Further, because the nadir GFR can be multifactorial and difficult to predict, the greatest benefit to nephron sparing may be in those with already compromised renal function. Towards this, enucleative and unclamped techniques may have a specific role in optimizing post-operative renal function in these scenarios (39). Further, the majority of data demonstrating that CKD has an increased risk of progression to end-stage renal disease, cardiac morbidity and even death is due to long-standing medical comorbidities such as diabetes and not surgically-induced causes of CKD. Loss of nephrons due to surgical resection may be associated with a decreased likelihood of CKD progression, relative to those with medically induced CKD (40,41). These data support the notion surgically induced CKD is less harmful than medical CKD, and since patients with CKD are at the greatest risk of further renal function decline with surgery, PN should be favored (42). Nevertheless, PN is not without morbidity and an earlier reporting of the findings from the EORTC-GU noninferiority phase 3 trial 30904 showed an unanticipated OS benefit for RN (4). These level 1 results, although not necessarily reflective of contemporary PN, should continued to be weighed in the decision making process for patients with marginal renal function.
Other limiting factors
The decision to perform a PN has largely been dependent on the location, complexity, and size of the renal mass. There has been increasing adoption of renal nephrometry systems such as the RENAL score, PADUA prediction score, and centrality index (C-index) to assist in determining the complexity of the PN and the likelihood of complications (43,44). While these factors are critical for determining surgical approach, there are additional anatomic and surgical restraints that can dictate the feasibility of PN. The quantity and the quality of the perinephric fat can influence the technical difficulty of a PN. Much time can be allotted to removing adherent perinephric adipose tissue in preparation for a PN. It is this fat and not necessarily body mass index that is more likely to lead to poor surgical exposure during hilar dissection, tumour excision, and renorrhaphy (45). Recently, Davidiuk et al. introduced an image-based scoring system, the Mayo Adhesive Probability (MAP), to predict intraoperative adherent perinephric fat, based on posterior perinephric fat thickness and stranding (46). The anticipation of “sticky” fat would allow surgeons to counsel patients on predicted anatomical challenges during PN and the possibility of conversion to RN.
PN in the setting of prior renal surgery represents a potential limit to the application of NSS. In light of the fact recurrences may be related to the multifocality of renal masses, RN has traditionally been viewed as the optimal surgical strategy, however, the goal of nephron preservation has gained increased traction (47). Multiple, single-institutional experiences with PN in the setting of previous renal surgeries have been reported with acceptable peri-operative outcomes despite the challenging nature of these procedures (Table 1) (48-54).
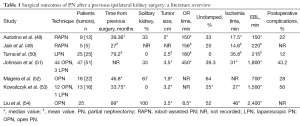
Full table
Ablative procedures such as radiofrequency ablation and cryotherapy are increasingly utilized in the management of SRMs, particularly in non-surgical candidates (55-57). However, when ablation is unsuccessful and/or recurrence is identified, salvage surgery typically entails RN, although several series have demonstrated the possibility of PN, albeit representing very challenging surgical scenarios. Zermann et al. reviewed the Cleveland Clinic experience with attempted PN after either radiofrequency ablation or cryotherapy (58). In this small series, only two of the ten patients underwent successful PN on account of significant perinephric fibrosis whereas the remainder underwent RN or abortion of the procedure. A series from the National Cancer Institute reported more successful outcomes for PN after radiofrequency ablation (53). Most of these patients had severe fibrosis, but PN was completed in all patients (n=16) and required a prolonged operative time together with a greater risk of transfusion. In this series, there was a moderate increase in the risk of complications such as prolonged urine leak and the need for re-operation. The MD Anderson Cancer Center experience with salvage renal surgery following energy ablation was recently reported (59). Of 14 patients, 11 underwent PN while the remainder underwent planned RN. The procedures were technically difficult with two patients requiring intraoperative transfusions, together with the potential need for aggressive local resection to achieve negative margins (e.g., resection of the psoas muscle). Taken together, these studies suggest that, in the appropriately selected patients, PN is feasible despite being technically demanding.
Future directions
The increased utilization of abdominal imaging has amplified the incidental detection of SRMs. Of the 64,000 new masses in 2012, nearly 74% of them were SRMs (4 cm or less), and a substantial number of them were benign (20-30%) (60). Accurate characterization of these masses is necessary to guide treatment, including potentially avoiding intervention for benign lesions. Radiologic assessment and needle biopsy are currently used to better characterize SRMs, however, both of these approaches have limitations. Radiologic imaging has little value in predicting small renal mass growth whereas needle biopsies of masses smaller than 3 cm have a high false negative rate.
We are currently investigating the utility of DNA methylation markers from tissue obtained from needle biopsies to improve the diagnostic accuracy and gain prognostic information for SRMs. Our preliminary analysis has demonstrated that there are distinct methylation profiles for SRMs based on their histologic pathologies. When data from ex vivo needle biopsies is combined with data from The Cancer Genome Atlas (TCGA), the methylation profile of the specific histologic pathology appears to cluster together and can be used to differentiate one from another (61). Further investigations are underway to determine if methylation data provided from needle biopsies can play a role in the clinical management of patients with SRMs by detecting cancer at the early stages, reducing over-diagnosis and false positives, and accurately identifying non-malignant tumors. In addition to potentially avoiding aggressive treatment, a secondary goal is to identify patients that would most benefit from active surveillance.
Conclusions
Demonstration of safety, equivalent oncologic efficacy together with improved renal functional outcomes, has propelled PN as the standard of care for SRMs. There is increasing consideration of PN in the treatment of tumors of greater size, complexity as well as in locally advanced or cytoreductive scenarios. PN may also have a role in technically challenging scenarios of previous renal surgery or following failed renal mass ablation.
Acknowledgements
Funding: Research was supported by a grant from R21-CA167367 to G Liang, a NIH Exploratory/Developmental Research Grant Award. Funding period: 2012-07-10 to 2015-06-30.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer 2009;115:1465-71. [PubMed]
- Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 2008;179:468-71; discussion 472-3. [PubMed]
- Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol 2009;181:55-61; discussion 61-2. [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [PubMed]
- Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000;75:1236-42. [PubMed]
- McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002;59:816-20. [PubMed]
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735-40. [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [PubMed]
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271-9. [PubMed]
- Ljungberg B, Bensalah K, Bex A, et al. Guidelines on Renal Cell Carcinoma. In: European Association of Urology Web site, 2015. Available online: http://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR.pdf
- Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol 2007;177:70-4; discussion 74. [PubMed]
- Permpongkosol S, Bagga HS, Romero FR, et al. Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol 2006;176:1984-8; discussion 1988-9.
- Russo P. End stage and chronic kidney disease: associations with renal cancer. Front Oncol 2012;2:28. [PubMed]
- Malcolm JB, Bagrodia A, Derweesh IH, et al. Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int 2009;104:476-81. [PubMed]
- Bagrodia A, Kopp RP, Mehrazin R, et al. Impact of renal surgery for cortical neoplasms on lipid metabolism. BJU Int 2014;114:837-43. [PubMed]
- Alanee S, Nutt M, Moore A, et al. Partial nephrectomy for T2 renal masses: contemporary trends and oncologic efficacy. Int Urol Nephrol 2015;47:945-50. [PubMed]
- Thompson RH, Kaag M, Vickers A, et al. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol 2009;181:993-7. [PubMed]
- Dash A, Vickers AJ, Schachter LR, et al. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int 2006;97:939-45. [PubMed]
- Crépel M, Jeldres C, Perrotte P, et al. Nephron-sparing surgery is equally effective to radical nephrectomy for T1BN0M0 renal cell carcinoma: a population-based assessment. Urology 2010;75:271-5. [PubMed]
- Antonelli A, Ficarra V, Bertini R, et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: results of a retrospective, comparative, multi-institutional study. BJU Int 2012;109:1013-8. [PubMed]
- Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology 2009;73:1077-82. [PubMed]
- Hansen J, Sun M, Bianchi M, et al. Assessment of cancer control outcomes in patients with high-risk renal cell carcinoma treated with partial nephrectomy. Urology 2012;80:347-53. [PubMed]
- Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int 2014;114:708-18. [PubMed]
- Breau RH, Crispen PL, Jimenez RE, et al. Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J Urol 2010;183:903-8. [PubMed]
- Long CJ, Canter DJ, Kutikov A, et al. Partial nephrectomy for renal masses ≥ 7 cm: technical, oncological and functional outcomes. BJU Int 2012;109:1450-6. [PubMed]
- Gill IS, Metcalfe C, Abreu A, et al. Robotic Level III Inferior Vena Cava Tumor Thrombectomy: The initial series. J Urol 2015. [Epub ahead of print]. [PubMed]
- Al Otaibi M, Abou Youssif T, Alkhaldi A, et al. Renal cell carcinoma with inferior vena caval extention: impact of tumour extent on surgical outcome. BJU Int 2009;104:1467-70. [PubMed]
- Margulis V, Tamboli P, Jacobsohn KM, et al. Oncological efficacy and safety of nephron-sparing surgery for selected patients with locally advanced renal cell carcinoma. BJU Int 2007;100:1235-9. [PubMed]
- Babaian KN, Merrill MM, Matin S, et al. Partial nephrectomy in the setting of metastatic renal cell carcinoma. J Urol 2014;192:36-42. [PubMed]
- Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704-10. [PubMed]
- Krambeck AE, Leibovich BC, Lohse CM, et al. The role of nephron sparing surgery for metastatic (pM1) renal cell carcinoma. J Urol 2006;176:1990-5; discussion 1995.
- Capitanio U, Zini L, Perrotte P, et al. Cytoreductive partial nephrectomy does not undermine cancer control in metastatic renal cell carcinoma: a population-based study. Urology 2008;72:1090-5. [PubMed]
- Hellenthal NJ, Mansour AM, Hayn MH, et al. Is there a role for partial nephrectomy in patients with metastatic renal cell carcinoma? Urol Oncol 2013;31:36-41. [PubMed]
- Patel AR, Eggener SE. Warm ischemia less than 30 minutes is not necessarily safe during partial nephrectomy: every minute matters. Urol Oncol 2011;29:826-8. [PubMed]
- Lane BR, Russo P, Uzzo RG, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol 2011;185:421-7. [PubMed]
- Campbell SC. A nonischemic approach to partial nephrectomy is optimal. No. J Urol 2012;187:388-90. [PubMed]
- Simone G, Gill IS, Mottrie A, et al. Indications, Techniques, Outcomes, and Limitations for Minimally Ischemic and Off-clamp Partial Nephrectomy: A Systematic Review of the Literature. Eur Urol 2015. [Epub ahead of print]. [PubMed]
- Gill IS, Eisenberg MS, Aron M, et al. “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol 2011;59:128-34. [PubMed]
- Satkunasivam R, Tsai S, Syan S, et al. Robotic Unclamped ‘Minimal-margin’ Partial Nephrectomy: Ongoing Refinement of the Anatomical Zero-ischemia Concept. Eur Urol 2015. [Epub ahead of print].
- Lane BR, Campbell SC, Demirjian S, et al. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 2013;189:1649-55. [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Is all chronic kidney disease created equal? Curr Opin Urol 2014;24:127-34. [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol 2015. [Epub ahead of print]. [PubMed]
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. [PubMed]
- Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol 2010;183:1708-13. [PubMed]
- Ioffe E, Hakimi AA, Oh SK, et al. Effect of visceral obesity on minimally invasive partial nephrectomy. Urology 2013;82:612-8. [PubMed]
- Davidiuk AJ, Parker AS, Thomas CS, et al. Mayo adhesive probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol 2014;66:1165-71. [PubMed]
- Shuch B, Linehan WM, Bratslavsky G. Repeat partial nephrectomy: surgical, functional and oncological outcomes. Curr Opin Urol 2011;21:368-75. [PubMed]
- Autorino R, Khalifeh A, Laydner H, et al. Repeat robot-assisted partial nephrectomy (RAPN): feasibility and early outcomes. BJU Int 2013;111:767-72. [PubMed]
- Jain S, Yates JK, Munver R. Robot-assisted laparoscopic partial nephrectomy for recurrent renal-cell carcinoma in patients previously treated with nephron-sparing surgery. J Endourol 2013;27:309-12. [PubMed]
- Turna B, Aron M, Frota R, et al. Feasibility of laparoscopic partial nephrectomy after previous ipsilateral renal procedures. Urology 2008;72:584-8. [PubMed]
- Johnson A, Sudarshan S, Liu J, et al. Feasibility and outcomes of repeat partial nephrectomy. J Urol 2008;180:89-93; discussion 93. [PubMed]
- Magera JS Jr, Frank I, Lohse CM, et al. Analysis of repeat nephron sparing surgery as a treatment option in patients with a solid mass in a renal remnant. J Urol 2008;179:853-6. [PubMed]
- Kowalczyk KJ, Hooper HB, Linehan WM, et al. Partial nephrectomy after previous radio frequency ablation: the National Cancer Institute experience. J Urol 2009;182:2158-63. [PubMed]
- Liu NW, Khurana K, Sudarshan S, et al. Repeat partial nephrectomy on the solitary kidney: surgical, functional and oncological outcomes. J Urol 2010;183:1719-24. [PubMed]
- Hinshaw JL, Lee FT Jr. Image-guided ablation of renal cell carcinoma. Magn Reson Imaging Clin N Am 2004;12:429-47. vi. [PubMed]
- Lowry PS, Nakada SY. Renal cryotherapy: 2003 clinical status. Curr Opin Urol 2003;13:193-7. [PubMed]
- Murphy DP, Gill IS. Energy-based renal tumor ablation: a review. Semin Urol Oncol 2001;19:133-40. [PubMed]
- Nguyen CT, Lane BR, Kaouk JH, et al. Surgical salvage of renal cell carcinoma recurrence after thermal ablative therapy. J Urol 2008;180:104-9; discussion 109. [PubMed]
- Karam JA, Wood CG, Compton ZR, et al. Salvage surgery after energy ablation for renal masses. BJU Int 2015;115:74-80. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Alemozaffar M, Coolings C, Chopra S, et al. MP29-13 improving renal needle-biopsy accurracy with rcc-specific dna methylation markers. Journal of Urology 2014;191:e309-10.


