Risk based neoadjuvant chemotherapy in muscle invasive bladder cancer
Introduction
According to Surveillance, Epidemiology, and End Results Program (SEER) estimates, there were over 74,000 new cases of bladder cancer and greater than 15,000 associated deaths in 2014, which remains largely unchanged over the last 25 years (1). Of these patients, 30% have muscle invasive bladder cancer (MIBC) at presentation and another 10% will progress from non-muscle invasive tumors. Radical cystectomy (RC) is the established standard of care for organ-confined tumors and has proven efficacy with extended follow-up cohorts reporting 5-year disease free survival from 68-85% (2-4). However, patient survival diminishes with increasingly advanced primary tumors, with a steep drop off once the cancer becomes non-organ confined or metastatic. Since currently available salvage therapies have very low rates of durable responses, with the notable exception of the recently FDA approved MPDL3280A (5), efforts to increase the success of definitive treatment have led to the utilization of perioperative chemotherapy.
Neoadjuvant chemotherapy (NC) has emerged as the preferred modality of delivering systemic therapy to non-metastatic MIBC patients planning to undergo RC. Based on evidence that will be enumerated below, NC provides a statistically significant survival benefit to patients. The benefit, however, is modest and the toxicities are prevalent, which has resulted in infrequent use of NC because of the perceived high risk (HR) and low benefit of the therapy (6). In this environment, there is an increasing demand to develop strategies that inform medical decision making to ensure those who require more aggressive therapies receive them. This article will review current and ongoing research on risk-stratified methods of identifying ideal candidates for NC.
The Case for NC
The question of perioperative chemotherapy was first addressed in the adjuvant setting with patients with extravesical disease or lymph node metastasis. A prospective trial from Skinner et al., demonstrated that adjuvant chemotherapy (AC) could improve relapse free survival in cystectomy patients from 46% to 70% (7). While there were subsequent randomized trials that confirmed this finding (8,9), there were still others with negative findings (10-12). Ruggeri et al. performed a pooled analysis of published phase III trials (n=5) and found that both overall survival (OS) and disease-free survival (DFS) were improved by the use of AC [response rate (RR) 0.74 and 0.65, respectively, P≤0.001] (13). Vale et al. further expanded on these results by performing a meta-analysis of the individual patient data from these randomized controlled trials (n=6) and corroborated the previous findings, showing a 25% risk reduction for OS, albeit with limited power (14). The recently published results of EORTC 30994, which compared immediate to salvage chemotherapy, appear to challenge the previous conclusions, as they did not demonstrate an OS advantage (47% vs. 57% mortality, respectively, P=0.13), suggesting that timing is not important for survival (15). However, the authors note that despite limited power for OS outcomes, progression free survival (PFS) was significantly improved (OR 0.54 for immediate), and there may be subgroups that can benefit from immediate AC. These studies demonstrate the importance of multimodal therapy in improving survival outcomes for patients with a poor prognosis with surgery alone.
In nearly all of these trials, a significant portion of the study population did not receive the complete AC regimen, which contributed to the lack of survival advantage in some trials. The low AC completion rate is, in part, attributed to the well-known high morbidity of RC, with quoted complication rates as high as 64% (13% high grade complications) (16). Donat et al. further examined this concept in a comprehensive examination of complication profiles among RC patients, and concluded that up to 30% of patients would be unable to receive timely AC due to prolonged recuperation (17). Additionally, the toxicity of the AC regimen is known to be particularly severe (18,19), which is compounded with the recovering state of post-operative patients resulting in as few as 56% of patients getting complete therapy in contemporary series (20). These results suggest that administering chemotherapy prior to RC might be a more favorable strategy to ensure patients are able to receive a complete chemotherapy course within the perioperative period.
There are several potential advantages of using NC instead of AC. Whereas extended surgical recovery precludes many patients from receiving or completing AC, giving chemotherapy up front when patients are at their optimal performance status increases the chance they receive the full dose/course of NC. The possibility of a complete tumor response, with the associated dramatic increase in survival, is the most compelling argument for NC. Nodal downstaging is another desirable outcome since occult lymph node metastasis is seen in 30-40% of cases, most likely due to micrometastatic disease not visualized on routine radiologic imaging (21). Finally, the degree of tumor response to NC gives a measure of in vivo drug sensitivity, which may also provide information on prognosis and choice of adjuvant/salvage therapy.
Evidence for NC
Through numerous prospective clinical trials, it has been determined that the ideal regimen for bladder cancer includes cisplatinum, and that replacement with other platinum based agents was not sufficient (22,23). The combination of methotrexate, vinblastine, adriamycin and cisplatinum (MVAC), initially described by Sternberg et al., has been shown to be the most effective regimen for bladder cancer, with overall RR of 65-72% in the metastatic setting (24,25).
Millikan et al. designed one of the early trials examining whether NC was a viable treatment alternative by comparing NC plus AC to AC alone, using the MVAC regimen (26). Although they did not demonstrate a survival benefit in this study, subgroup analysis showed that those rendered pT0 derived significant benefit. Based on these results, the utility for NC became more evident in this patient population. In the landmark SWOG-8710 study, Grossman et al. demonstrated that NC utilizing MVAC increased median survival from 46 to 77 months, and enhanced pathologic downstaging, with pT0 seen in 15% and 38% of RC and NC + RC patients, respectively (27). Griffiths et al. reported that cisplatin, methotrexate and vinblastine (CMV) could also produce a 16% reduction of the risk of death, with long term follow-up (median 8 years) (28). The analysis of the two Nordic trials by Sherif et al., demonstrated that even when combined with preoperative radiotherapy, cisplatin based regimens yielded at 20% relative and an 8% absolute risk reduction in death (29). Schultz et al. defined the importance of pre and post NC tumor stage in predicting survival, and confirmed that NC improves outcomes in patients with tumor downstaging (30). In subsequent meta-analyses of NC trials, it was shown that an absolute 5-6.5% OS benefit is observed when using MVAC NC for MIBC (31,32). The efficacy of this regimen is, unfortunately, tempered by an unfavorable toxicity profile, with documented granulocytopenia (33% grade 4) and gastrointestinal complications (17% grade 3-4) (27).
To mitigate these adverse effects, alternate regimens have been developed that retain the same efficacy of MVAC. Some centers have recently modified the standard 4 week cycle, to a 2-week cycle with granulocyte colony-stimulating factor (G-CSF) support, known as dose dense MVAC (ddMVAC) (33). Follow-up clinical trials in the neoadjuvant setting have demonstrated that ddMVAC results in effective RR (pT0 =26-38%), far less toxicity (0-10% grade 3-4) and more patients completing the full course (93-95% completion) (34,35). More popular is the combination of gemcitabine and cisplatinum (GC) which was shown by von der Maase et al. in a phase 3 study to have similar outcomes compared with MVAC, but with more tolerable toxicity (36). However, since the design of this trial was to establish superiority, not equivalence, the evidence does not strictly support the widespread use of GC as an alternative to MVAC. Zargar et al. recently published a multicenter retrospective study that compared GC to MVAC, with two important findings; GC was utilized in the majority of patients (64%) and no significant difference was seen in pathologic RR or OS (37). Regardless of the regimen used, NC has level 1 evidence to support its use (Table 1), which is reflected in published guidelines that recommend offering NC to MIBC patients who will be treated with RC (38,39).
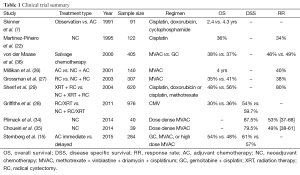
Full table
Are all MIBC patients equally responsive to NC?
There is a growing utilization of NC, with Zaid et al. reporting an increase from 7.6% to 20.9% over a 4-year period, but this still represents a minority of patients (40). The biggest factor behind the limited application of NC for RC candidates is the modest OS benefit observed in clinical trials, which is contrasted with notable toxicity and potential for delaying surgery in chemotherapy non-responders. Scrutinizing the data lends credence to this view and reveals that the survival advantage is largely seen in responders. Unfortunately, only 30% of patients achieve a complete response, and another 44% will have some degree of downstaging. This leaves the majority of patients over treated if NC was offered to all candidates. Additionally, when looking at the RC only arm, there is a 15% pT0 RR demonstrating that a complete TURBT may be sufficient to render these patients downstaged and likely accounts for half of the downstaging seen with NC (27). The survival outcomes of these patients are similar to those of NC pT0 patients, 82% and 85% 5-year OS, respectively (41,42). These results clearly point to the fact that only a certain subset of MIBC patients will respond favorably to cisplatin based NC.
To understand which patients most likely benefit from NC, Sonpavde et al. examined the patterns of survival and relapse from the SWOG-8710 study (43). Firstly, they observed that the RR was dependent on the baseline clinical stage, with cT2 patients downstaged 55% of the time, and cT3/4 patients 35% of the time. Similarly, the RR for pT0 was also stage dependent, 39% and 24% for cT2 and cT3/4, respectively. They also found that survival was dependent on the degree of response (median survival pT0 =13.6 years, pT1/a/is =10.6 years). Conversely, very poor outcomes were seen in patients who had no response or progression of disease (median survival of 3.7 years for pT2+). Paradoxically, when compared survival of non-responding cT2 and cT3/4 tumors (median survival of 1.8 and 5.1 years, respectively), the advanced clinical stage has a better prognosis. Overall, this report highlights that the baseline clinical stage is inversely related to the likelihood of response and that the final pathologic stage is prognostic of survival.
Risk factors
In order to selectively administer NC to the patients that will derive some benefit, researchers have tried to determine if there are preoperative factors that can be used to predict which patients will have the poorest outcomes. These can be roughly divided into those factors that represent locally advanced disease [palpable or fixed mass on examination under anesthesia (EUA)], cross-sectional imaging revealing signs of extravesical extension or local organ involvement, hydronephrosis) and those factors that predict regional/distant metastasis [lymphovascular invasion (LVI), and variant histology] (30,44-50).
Locally advanced disease is challenging to accurately diagnose, but has a significant impact on outcomes. The utility of a good physical exam can never be underestimated, and the presence of a 3-dimensional palpable mass on bimanual EUA is consistent with a cT3b stage, and if that mass is fixed, cT4b (51). Cross-sectional imaging is important for local and distant staging in any malignancy, however computed tomography (CT) imaging has a limited efficacy in bladder cancer, plagued by poor accuracy (49-55%) and high interobserver variability (κ=0.23-0.35) (52,53). Dynamic magnetic resonance imaging (MRI) has proven itself to have moderate staging accuracy (62-63%), and good ability to distinguish organ confined from locally advanced disease (82-90%) with strong interobserver agreement (κ=0.80-0.89) (54,55). Hydronephrosis has been proven to be a surrogate for invasive disease for many years (46), with risk increasing from unilateral to bilateral involvement (90%≥ pT3 with bilateral). Bartsch et al. showed in their study that hydronephrosis was an independent predictor of recurrence free survival (χ2=10.1, P=0.0015) (56). In early trials with bladder sparing tri-modal therapy, it was quickly determined that patients with hydronephrosis had such an abysmal success rate, that it is now considered a standard exclusion criteria (57). Currently, the best predictive information on extravesical disease comes from a combination of physical exam and radiologic imaging.
Metastatic disease, either to the regional lymph nodes or to distant sites, portends the worst prognosis, and yet, in the absence of measurable disease, there are only a few options to help guide clinicians. LVI is the strongest pre-surgical predictor of poor outcomes, able to independently predict OS, disease specific survival (DSS), recurrence (local and distant) in pN0 patients (58,59). There is data that suggests that the presence of LVI may predict failure of MVAC AC to improve outcomes in organ confined, node negative patients (60). Variant histology in bladder cancer includes many subtypes, but the variants that are of interest regarding early metastasis are micropapillary, small cell/neuroendocrine and plasmacytoid. Micropapillary is likely underreported due to interobserver variability both in academic institutions and community practice (61,62), but it is universally accepted that invasive micropapillary disease is associated with a higher incidence of extravesical and metastatic disease, and poor OS (63,64). While there is some data suggesting that NC may have efficacy in this group, due to small sample sizes, no definitive recommendation can be made (65). Small cell or neuroendocrine histology is another urothelial variant that has a grim prognosis. The largest series is from MD Anderson, with 172 patients in the cohort, with 50% of RC candidates receiving NC. NC has been shown to have a dramatic effect in this disease, with 62% downstaged to ≤ pT1 and a median OS improvement from 18.3 to 159.5 months (66). Plasmacytoid is a very rare and extremely aggressive variant of bladder cancer that has a predilection for peritoneal metastasis (67,68). Dayyani et al. reported a series of 31 patients (median OS, 17.7 months) in whom 5 received NC, with 80% downstaging, but with early relapse and no demonstrable difference between upfront surgery (69). In total, these are the only significant clues available that suggest early metastasis.
Recently, Culp et al. described the MD Anderson paradigm of classifying MIBC patients as low risk (LR) or HR based on these well-established factors, and correlated risk of upstaging and survival outcomes with RC alone (70). The classifiers of HR status are a palpable/fixed mass on EUA, radiologic evidence of cT3/4, the presence of hydronephrosis, LVI and variant histology. By analyzing their own series and using an external validation cohort, they found that LR patients had a 5-year OS and DSS of 64.8% and 82.7%, respectively, and 5-year OS and DSS for HR patients were dramatically worse at 47% and 68.2%. The surprising finding is that the risk of upstaging in the LR cohort was 49.2%, but the group overall had reasonable outcomes. Looking at the HR category, those patients that were downstaged to LR on final pathologic staging (26.5%), had a 5-year OS and DSS of 85.1% and 91%. These same findings were corroborated by the external validation cohort, which had a much larger sample size. This schema of risk assignment gives NC to those patients at the highest risk of poor outcomes, while allowing LR patients to be promptly treated with RC, and avoid NC toxicities.
Molecular classification of MIBC
There has long been an effort to characterize bladder cancer using molecular markers that represent the underlying biologic processes driving the disease course. The prototypical molecular target in bladder cancer was p53, which was identified in the early 1990’s as being correlated with grade, stage and risk of tumor progression (71-73). A follow-up study was performed at Memorial Sloan Kettering using immunohistochemistry on patient samples from an NC MVAC trial, which revealed that nuclear accumulation of p53 was independently predictive of DSS, with a relative risk of 3.1 (74). Unfortunately, conflicting reports afterwards have led to an indefinite determination on whether p53 is truly a biomarker of survival (75). The robustness of the molecular findings was limited by the technology available at the time, and may account for the variable results generated by the different study groups.
With the advent of next generation sequencing (NGS) and high throughput microarrays coupled with bioinformatics techniques, genomics research has been able to make large leaps in the discovery and understanding of the mechanisms of oncogenesis. In early 2014, The Cancer Genome Atlas (TCGA) Research Network released the results of their whole exome sequencing and whole genome expression profiling analysis of MIBC (76). There were significant alterations in 32 somatic genes, including TP53, RB1, FGFR3, EGFR, PPARγ and many others. Unsupervised hierarchical clustering of the sequencing data yielded three intrinsic molecular subtypes; group A enriched with copy number alterations, group B mainly comprised of papillary histology and FGFR3 alterations, and group C enriched with TP53 and RB1 mutations. In addition to this the TCGA, in parallel with groups at MD Anderson Cancer Center and University of North Carolina, used similar clustering techniques to identify molecular subtypes based on with gene expression data (77,78). While there are important differences between the classification systems, in general, they were able to mirror the pattern seen in breast cancer, identifying basal and luminal subtypes that are enriched with gene sets that reflect the milieu of the different lineages. Damrauer et al. demonstrated that cluster K1 expressed high molecular weight keratins and CD44, which are seen in basal cells, and cluster K2 expresses low molecular weight keratins and uroplakins, both seen in urothelial umbrella cells. When correlating the subtypes with clinical outcomes, they observed that basal tumors had poorer survival compared with luminal tumors. Choi et al. identified a similar basal/luminal dichotomy, with basal enrichment of p63 and squamous differentiation and luminal tumors with PPARγ. Additionally, they identified a subset within the luminal subtype that was characterized as “p53-like” and displayed significant platinum chemoresistance, both in the clinical cohort and in subsequent cell line studies. In addition to setting a benchmark for comprehensive genomic analysis of bladder cancer, these groups have established a classification framework that researchers can continue to refine.
Using similar techniques, other groups have correlated genomic findings to clinical outcomes that may inform patient management. Turo et al. created a tissue microarray using samples from the primary tumor and metastatic lymph nodes in patients that were clinically node negative prior to RC (79). Examining FGFR3 specifically, the authors found that there was a high concordance between the specimens (OR 8.45), even when using multiple samples from each site to account for intratumoral heterogeneity. This suggests that FGFR3 protein expression in the primary tumor can be used to identify patients at a HR of occult lymph node metastasis, and candidates for NC. Groenendijk et al. used NGS methods to compare the mutational profile of complete responders and non-responders to NC (80). Their group found that ERBB2 activating mutations were exclusively found in the responder cohort, with none present in the non-responders (P=0.003). ERCC2 mutations also appeared to be differentially expressed with 16% of responders and 6% of non-responders having the mutation, however this was not significant (P=0.27). Van Allen et al., however, performed whole exome sequencing on patients receiving cisplatin-based NC and demonstrated that ERCC2 mutations were enriched in responders (81). Further in vitro work demonstrated that ERCC2 deficient cell lines increase cisplatin sensitivity, and this effect is rescued with wildtype ERCC2, but not the mutant form found in the patient cohort. Font et al. analyzed gene expression in NC patients and found that high BRCA1 expression in pre-treated tumors predicted lower NC response (22% vs. 66%, P=0.01) and lower OS (HR 2.73, P=0.02) (82). Using molecular characteristics to identify patients with HR disease or to predict patients likely to respond to NC, the major contribution of these efforts is that this information is correlated to a meaningful difference in clinical behavior, demonstrating the importance of translational research.
Conclusions
If we could perfectly identify responders to NC, or if the toxicities were minimal, utilization rates would be much higher than they are now. Unfortunately, neither of those conditions is currently true. We now know that there are factors that we can use to stratify patients into high and LR. Even with a high incidence of upstaging amongst LR patients, it has been shown that their outcomes with RC alone are similar to patients that had no stage change. But this binary system is still a relatively unsophisticated way of guiding decision making. Knowing that we can identify patients who do well with surgery alone, we now need to identify which HR patients will respond well to cisplatin based NC, and those who need alternate treatments based on novel targets.
New molecular classifiers are being created to characterize tumors based on the underlying cancer biology, with important implications concerning progression and chemoresistance. This is certainly the direction that bladder cancer research needs to follow, in order to refine decision making to attain the goal of personalized medicine. Already several genomic classifiers have been developed, which have been designed for predicting DSS after cystectomy. The most recent, developed by Mitra et al., is a 15-gene classifier that predicts recurrence after RC without NC, with superior performance compared with currently available clinical predictors. In a more prospective fashion, the recently activated SWOG-NCI sponsored COXEN clinical trial (S1314) plans to compare GC and MVAC NC, and simultaneously collect tissue, blood and urine samples to process through the COXEN algorithm (83). The COXEN algorithm has already been able to develop and validate a multivariate gene expression model for survival in NC treated bladder cancer, using a combination of publicly available human microarray data sets and in vitro drug sensitivity testing using the NCI-60 cell line panel (84). In the current prospective trial, gene sequencing and expression profiling will be performed to analyze oncogenomics, expression patterns of coding and non-coding RNA, and pharmacogenomics. Instead of simply comparing two different NC regimens, this trial is unique in the fact that it will allow investigators to discover patterns of sensitivity/resistance and develop molecular signatures to guide decision making in multimodality cancer treatment. With the initiation of more trials like this, we will be able to test new therapeutic agents, and ideally be able to predict the right drug for the right patient at the right time.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute, 2012.
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [PubMed]
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414-22; discussion 2422. [PubMed]
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012;61:1039-47. [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [PubMed]
- Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol 2015;67:165-70. [PubMed]
- Skinner DG, Daniels JR, Russell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol 1991;145:459-64; discussion 464-7. [PubMed]
- Lehmann J, Franzaring L, Thüroff J, et al. Complete long-term survival data from a trial of adjuvant chemotherapy vs control after radical cystectomy for locally advanced bladder cancer. BJU Int 2006;97:42-7. [PubMed]
- Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol 1996;155:495-9; discussion 499-500. [PubMed]
- Studer UE, Bacchi M, Biedermann C, et al. Adjuvant cisplatin chemotherapy following cystectomy for bladder cancer: results of a prospective randomized trial. J Urol 1994;152:81-4. [PubMed]
- Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol 2011;29:3443-9. [PubMed]
- Cognetti F, Ruggeri EM, Felici A, et al. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 2012;23:695-700. [PubMed]
- Ruggeri EM, Giannarelli D, Bria E, et al. Adjuvant chemotherapy in muscle-invasive bladder carcinoma: a pooled analysis from phase III studies. Cancer 2006;106:783-8. [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol 2005;48:189-99; discussion 199-201. [PubMed]
- Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76-86. [PubMed]
- Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164-74. [PubMed]
- Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol 2009;55:177-85. [PubMed]
- Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 1990;8:1050-5. [PubMed]
- Logothetis CJ, Finn LD, Smith T, et al. Escalated MVAC with or without recombinant human granulocyte-macrophage colony-stimulating factor for the initial treatment of advanced malignant urothelial tumors: results of a randomized trial. J Clin Oncol 1995;13:2272-7. [PubMed]
- Dorff TB, Tsao-Wei D, Miranda G, et al. Adjuvant chemotherapy for locally advanced urothelial carcinoma: an overview of the USC experience. World J Urol 2009;27:39-44. [PubMed]
- Mertens LS, Meijer RP, Meinhardt W, et al. Occult lymph node metastases in patients with carcinoma invading bladder muscle: incidence after neoadjuvant chemotherapy and cystectomy vs after cystectomy alone. BJU Int 2014;114:67-74. [PubMed]
- Martinez-Piñeiro JA, Gonzalez Martin M, Arocena F, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: a prospective randomized phase III study. J Urol 1995;153:964-73. [PubMed]
- Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 1997;80:1966-72. [PubMed]
- Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403-7. [PubMed]
- Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 1989;64:2448-58. [PubMed]
- Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 2001;19:4005-13. [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [PubMed]
- International Collaboration of Trialists. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171-7. [PubMed]
- Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol 2004;45:297-303. [PubMed]
- Schultz PK, Herr HW, Zhang ZF, et al. Neoadjuvant chemotherapy for invasive bladder cancer: prognostic factors for survival of patients treated with M-VAC with 5-year follow-up. J Clin Oncol 1994;12:1394-401. [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [PubMed]
- Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol 2004;171:561-9. [PubMed]
- Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 2001;19:2638-46. [PubMed]
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895-901. [PubMed]
- Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 2014;32:1889-94. [PubMed]
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068-77. [PubMed]
- Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015;67:241-9. [PubMed]
- Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013;11:446-75. [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [PubMed]
- Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014;83:75-80. [PubMed]
- Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014;65:350-7. [PubMed]
- Tilki D, Svatek RS, Novara G, et al. Stage pT0 at radical cystectomy confers improved survival: an international study of 4,430 patients. J Urol 2010;184:888-94. [PubMed]
- Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 2009;115:4104-9. [PubMed]
- Herr HW. A proposed simplified staging system of invasive bladder tumors. Urol Int 1993;50:17-20. [PubMed]
- Yaman O, Baltaci S, Arikan N, et al. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: comparison with final pathological stage in invasive bladder carcinoma. Br J Urol 1996;78:197-200. [PubMed]
- Haleblian GE, Skinner EC, Dickinson MG, et al. Hydronephrosis as a prognostic indicator in bladder cancer patients. J Urol 1998;160:2011-4. [PubMed]
- Harada K, Sakai I, Hara I, et al. Prognostic significance of vascular invasion in patients with bladder cancer who underwent radical cystectomy. Int J Urol 2005;12:250-5. [PubMed]
- Quek ML, Stein JP, Nichols PW, et al. Prognostic significance of lymphovascular invasion of bladder cancer treated with radical cystectomy. J Urol 2005;174:103-6. [PubMed]
- Monn MF, Kaimakliotis HZ, Pedrosa JA, et al. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol 2015;33:18.e15-20.
- Soave A, Schmidt S, Dahlem R, et al. Does the extent of variant histology affect oncological outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy? Urol Oncol 2015;33:21.e1-9.
- Urinary bladder. In: Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging handbook seventh edition. London: Springer, 2010:569.
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol 2000;163:1693-6. [PubMed]
- Tritschler S, Mosler C, Tilki D, et al. Interobserver variability limits exact preoperative staging by computed tomography in bladder cancer. Urology 2012;79:1317-21. [PubMed]
- Rajesh A, Sokhi HK, Fung R, et al. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol 2011;66:1140-5. [PubMed]
- Tekes A, Kamel I, Imam K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol 2005;184:121-7. [PubMed]
- Bartsch GC, Kuefer R, Gschwend JE, et al. Hydronephrosis as a prognostic marker in bladder cancer in a cystectomy-only series. Eur Urol 2007;51:690-7;discussion 697-8. [PubMed]
- Shipley WU, Kaufman DS, Zehr E, et al. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology 2002;60:62-7; discussion 67-8. [PubMed]
- Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol 2005;23:6533-9. [PubMed]
- Canter D, Guzzo T, Resnick M, et al. The presence of lymphovascular invasion in radical cystectomy specimens from patients with urothelial carcinoma portends a poor clinical prognosis. BJU Int 2008;102:952-7. [PubMed]
- von Rundstedt FC, Mata DA, Groshen S, et al. Significance of lymphovascular invasion in organ-confined, node-negative urothelial cancer of the bladder: data from the prospective p53-MVAC trial. BJU Int 2014. [Epub ahead of print]. [PubMed]
- Sangoi AR, Beck AH, Amin MB, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol 2010;34:1367-76. [PubMed]
- Shah RB, Montgomery JS, Montie JE, et al. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: impact of mandatory central pathology review at a large referral hospital. Urol Oncol 2013;31:1650-5. [PubMed]
- Ghoneim IA, Miocinovic R, Stephenson AJ, et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 2011;77:867-70. [PubMed]
- Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007;110:62-7. [PubMed]
- Porten SP, Willis D, Kamat AM. Variant histology: role in management and prognosis of nonmuscle invasive bladder cancer. Curr Opin Urol 2014;24:517-23. [PubMed]
- Lynch SP, Shen Y, Kamat A, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol 2013;64:307-13. [PubMed]
- Keck B, Stoehr R, Wach S, et al. The plasmacytoid carcinoma of the bladder--rare variant of aggressive urothelial carcinoma. Int J Cancer 2011;129:346-54. [PubMed]
- Ricardo-Gonzalez RR, Nguyen M, Gokden N, et al. Plasmacytoid carcinoma of the bladder: a urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol 2012;187:852-5. [PubMed]
- Dayyani F, Czerniak BA, Sircar K, et al. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol 2013;189:1656-61. [PubMed]
- Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol 2014;191:40-7. [PubMed]
- Esrig D, Spruck CH 3rd, Nichols PW, et al. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol 1993;143:1389-97. [PubMed]
- Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259-64. [PubMed]
- George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol 2007;25:5352-8. [PubMed]
- Sarkis AS, Bajorin DF, Reuter VE, et al. Prognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVAC. J Clin Oncol 1995;13:1384-90. [PubMed]
- Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 2005;6:678-86. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [PubMed]
- Turo R, Harnden P, Thygesen H, et al. FGFR3 expression in primary invasive bladder cancers and matched lymph node metastases. J Urol 2015;193:325-30. [PubMed]
- Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol 2015. [Epub ahead of print].
- Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140-53. [PubMed]
- Font A, Taron M, Gago JL, et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann Oncol 2011;22:139-44. [PubMed]
- Lee JK, Havaleshko DM, Cho H, et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A 2007;104:13086-91. [PubMed]
- Williams PD, Cheon S, Havaleshko DM, et al. Concordant gene expression signatures predict clinical outcomes of cancer patients undergoing systemic therapy. Cancer Res 2009;69:8302-9. [PubMed]


