Active surveillance and focal therapy for low-intermediate risk prostate cancer
Introduction
The problem of prostate cancer overdiagnosis and overtreatment emerged shortly after PSA testing became widely adopted in North America and Europe beginning in the late 1980s. Enthusiasm for screening, despite evidence of overdiagnosis, continued unabated until 2012, when the US Preventive Services Task Force published a level D recommendation against screening (1), followed by several other respected national health policy organizations, including the Canadian Task Force on the Public Health Exam (CTFPHE) (2). The use of PSA for prostate cancer screening and early detection has declined over the last few years, reflecting the impact of these recommendations. Nonetheless, screening is still highly controversial.
The recommendations against PSA screening were largely due to concerns about overdiagnosis and overtreatment of non-clinically significant disease. As a result of these recommendations, the role of radical intervention for low risk cancer has been re-evaluated, and conservative management for low risk patients has been increasingly adopted by clinicians. The two approaches that reduce overtreatment and its attendant risks of adverse quality of life effects are active surveillance and focal therapy. They are complementary. Both share the principle that tissue preservation is important when it is possible. Men with lower risk disease can be managed with active surveillance, and defer treatment, in most cases for life. Men with higher risk disease that can be localized to a relatively small volume of the prostate may be candidates for focal therapy. The rationale and results of these two approaches will be reviewed in this article.
The natural history and molecular biology of low grade prostate cancer
Prostate cancer develops with age in the majority of men, including those from all races and regions. In Caucasians, the chance of harboring prostate cancer is approximately the same as one’s age; thirty percent of men in their 30’s, 40% in their 40’s, 80% in their 80s (3). Most of these are microfoci (<1 mm3) and low grade, particularly in younger men. The high prevalence of microfocal prostate cancer has been confirmed in autopsy studies of Caucasians, Asians, and other ethnic groups going back more than 50 years. A recent autopsy study in Japanese and Russian men who died of other causes showed that overall 35% of both groups had prostate cancer, and 50% of the cancers in Japanese men aged >70 were Gleason score 7 or above (3).
Genetic features of low grade prostate cancer
The two most common histologic patterns of prostate cancer are Gleason pattern 3 and 4. Importantly, as a result of several modifications of the Gleason system, a grade shift has occurred over the last 20 years. Many cases called Gleason 3 prior to 2005 are now called Gleason 7, particularly those with cribriform pattern histology. Thus Gleason 3 today is more ‘benign’ than in the past (4). The molecular hallmarks of cancer differ profoundly between pattern 3 and 4, and represents an important basis for the dramatically different approach taken to these two types of cancer (5,6). The six original hallmarks of cancer described by Hanahan and Weinberg include unlimited replicative potential, sustained angiogenesis, local tissue invasion, insensitivity to antigrowth signals, metastasis, and replicative self-sufficiency. More recently, de-regulated cellular energetics and evasion of immune destruction have been added to this list. The genetic pathways responsible for these hallmarks of malignancy have been worked out in detail (Table 1). The Gleason score has a remarkable ability to disaggregate prostate cancer between genetically relatively normal and abnormal cells. There are many examples of this. Genetic pathways mediating apoptosis resistance, angiogenesis and the development of other pro-angiogenic factors, genes involved in regulating cellular metabolomics, and metastasis and invasion processes, are similarly overexpressed in Gleason 4 and normal in 3 (7-18,21,24). Proliferation pathway associated genes, including AkT and HER2neu, are expressed normally in Gleason 3 and abnormally in Gleason 4 (Table 1). There are exceptions; in particular, both pTEN (19) and TMPRSS2-ERG (20,25), commonly up-regulated and present respectively in most Gleason 4s, are altered in a significant proportion of Gleason 3. The likelihood of a pTEN deletion is much higher in Gleason 3 from a prostate with co-existent Gleason 4, indicated that pre-histologic genetic changes occur. It is not surprising that some genetic heterogeneity exists within a single histologic pattern. However, these isolated genetic alterations do not appear to translate into an aggressive phenotype, with rare exceptions.
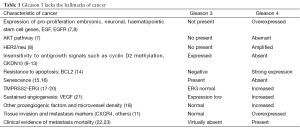
Full table
Metastatic potential
Several large clinical series have reported a rate of metastasis for surgically confirmed Gleason 6 (where there is no possibility of occult higher grade cancer co-existing in the prostate, and accounting for the metastasis) that approaches zero (22,23). Occult higher grade cancer is present in about 25% of patients whose biopsy shows only low grade cancer, and this likely accounts for the prostate cancer deaths reported in series of low risk patients managed conservatively
An alternative explanation for the exceptionally low rate of metastasis after surgery for Gleason 6 cancer is that the intervention is highly successful, and alters the natural history of the disease. However, if so, a reasonable expectation is that a few of the Gleason 6 cancers would have micro-metastasized prior to surgery, or to had a local recurrence with subsequent metastasis. This is rarely seen, if ever. An analogy is the results of surgical management of basal cell carcinomas of the skin, which are almost universally cured by surgical resection, and yet may become lethal if neglected. Further, if resection of a small basal cell carcinoma of the skin had the same effects on quality of life as a radical prostatectomy, dermatologists would plausibly also be advocating for conservative management in the ‘low risk’ cases!
One multi-center study of 24,000 men with long term follow-up after surgery included 12,000 with surgically confirmed Gleason 6 cancer (22). The 20-year prostate cancer mortality was 0.2%. A total of 4,000 of these were treated at MSKCC; of these, 1 died of prostate cancer; a pathological review of this patient revealed Gleason 4+3 disease (Scott. Eggener, personal communication). A second study of 14,000 men with surgically confirmed Gleason 6 disease found only 22 with lymph node metastases; review of these cases showed that all had higher grade cancer in the primary tumour. The rate of node positive disease in the patients with no Gleason 4 or 5 disease in their prostates was therefore zero (23). (In this study patients had, in most cases, a limited node dissection, and perhaps a more thorough resection might have identified more positive nodes; but the message is still unequivocal. Lymph node metastases with Gleason 6 are extremely rare).
Genetic analysis of cancer has demonstrated conclusively that pre-histologic mutations that confer an aggressive phenotype without altering the histology may occur. A recent genetic analysis of multiple metastatic sites from a patient who had extensive Gleason 4+3 pT3a N1 disease resected at age 47, and died 17 years later of metastatic CRPC, reported that the metastatic lesions appeared to derive from a microfocus of Gleason pattern 3 disease, rather than, as expected, from the high-grade cancers elsewhere in the prostate (26). This case report is a challenge to the view that Gleason pattern 3 does not metastasize. However, the report should be viewed in the context that it is a single case report; that the patient had Gleason 4+3 cancer with a small component of pattern 3, not Gleason 6 cancer which metastasized; and histological Gleason pattern 3, particularly when it coexists with higher grade cancer, may harbour pre-histological genetic alterations that confer a more-aggressive phenotype. It is has been proposed that in this case the low grade cancer occurred as a result of clonal re-differentiation from a higher grade cancer that metastasized, resulting in a common genetic phenotype (27). This is the conceptual basis for genetically based predictive assays that disaggregate low grade cancer into low and higher risk groups. Another case report from the same group described a patient on active surveillance who had annual biopsies for 12 years, all of which were negative or contained microfocal Gleason 6 cancer. Five years after the 12th biopsy the patient was found to have metastatic prostate cancer due to extensive Gleason 9 disease on repeat biopsy. Genetic characterization showed a complete switch of the molecular phenotype, indicating that the high grade cancer was unrelated to the original Gleason 6 disease (28).
Several new biomarkers have been approved by the FDA based on their ability to predict co- a higher risk of adverse pathologic findings in low grade prostate cancer patients. These include the Oncotype DX assay (Genome Health) which identifies a panel of genes linked to a more aggressive phenotype (29), and the Prolaris assay (30) (Myriad Genetics), which looks for abnormal expression of cell cycle related genes. The Decipher assay powerfully predicts for the risk of PSA failure after surgery (31). The Mitomics assay identifies the presence of a functional mitochondrial DNA deletion associated with aggressive prostate cancer (32). These tests interrogate the microfocus of Gleason 6 found on biopsy to identify the risk of those cells progressing to higher risk disease, as well as for the presence of higher grade cancer elsewhere in the prostate. That the biomarkers can achieve this confirms the inter-relationship of heterogeneous multi-focal cancers.
These molecular tests, performed on biopsy tissue, predict future biological behavior based on identifying genetic alterations in low-grade cancer cells. An unmet need is to better understand how to integrate the results of genetic biomarker tests and MRI. For example, the optimal management of the patient in whom results are discrepant (i.e., genetic test indicates high risk but MRI is negative) is currently unknown. Both diagnostic approaches are not perfect. MRI can miss small high grade cancers; the molecular assays may over or underestimate risk. These genetic tests are likely to be most useful for the low risk (higher volume of Gleason 6 than very low risk) and low-intermediate risk (small amount of Gleason 4 disease on biopsy) patients who wish a surveillance approach. A study of the impact of the Genomic Health GPS test showed that 25% of patients had their management changed as a result of the test, always in the direction suggested by the test result (33).
Multiparametric MRI is another powerful tool to identify the ‘wolf in sheep’s clothing’, that is, the patient with low grade cancer on biopsy who harbours a large high grade cancer elsewhere in the prostate (34). These occult cancers are usually anterior, and a part of the prostate that is harder to target using TRUS guided systematic biopsies, but easily seen on MRI. One study showed that 100% of cancers >1 cm in men on surveillance who subsequently had surgery were anterior (35).
Appreciating that Gleason pattern 3 has little or no metastatic phenotype has altered our approach to patients with this cancer. Phrases, like ‘pseudo-cancer’, ‘pseudo-disease’, ‘part of the aging process’, and ‘pre-cancer’, are useful in counseling these men. They are reassuring, and accurately reflect the extremely indolent nature of the disease entity. The capacity to metastasize is not the sine qua non of cancer, and local invasion, which can occur with Gleason 6, does legitimize the term ‘cancer’ for this disease. However, changing the terminology away from ‘cancer’, the diagnosis of which has profound emotional and psychological implications for patients, would significantly reassure the patient and derail a headlong rush into aggressive treatment.
Active surveillance is appropriate for men of all ages, including young men (under 55). The benefits of avoiding erectile dysfunction and incontinence are greater in young men, and the risks of second malignancies occurring in the irradiated field as sequelae of radiation are also greater in men with a long life expectancy. About 40% of men in their 40s harbor microfocal have low-grade cancer (36). Diagnosing this on a transrectal ultrasound (TRUS)-guided biopsy, does not mean that disease progression is inevitable. High volume Gleason 6 also does not preclude expectant management. However, those with high-volume Gleason pattern 3 have a considerably higher risk of harboring higher grade cancer. The volume threshold of Gleason 3 on biopsy at which point higher grade cancer is more likely to be present is not clear; it may be a continuous variable. One group recently identified this as more than 8 mm of total cancer on systematic biopsy (37). The management of patients with higher volume Gleason 6 is to rigorously exclude the presence of higher-grade cancer (based on MRI, targeted/template biopsies, and biomarkers). Patients confirmed to have only Gleason 6, even if higher volume, are unlikely to require treatment.
The benefits of PSA screening has been discounted by health policy bodies such as the USPSTF because of concerns about overtreatment and a high number needed to treat (NNT) for each death avoided. However, many believe that abandoning early detection will result in an increase in prostate cancer mortality. Selective treatment employing active surveillance would result in a decrease in the NNT for each death avoided. Thus, if widely adopted, active surveillance should eventually result in a re-appraisal of the benefits of PSA screening, and a greater acceptance of its value by policy makers such as the USPSTF. The result might be a re-acceptance of PSA screening, earlier identification of those with aggressive disease, lives saved, and an overall reduction in prostate cancer mortality (compared to no screening resulting from the perceived hazards of overtreatment). Less is more!
Who is a candidate for active surveillance? Low risk disease based on biopsy is widely defined as Gleason 6 and PSA <10 ng/mL. Most such newly diagnosed patients are stage T1c. This group includes around 45% of newly diagnosed patients in the USA and Canada, approximately 150,000 men per year. Low risk disease has been stratified into very low and low based on the number of cores, extent of core involvement, and PSA density (38). The Epstein criteria include patients with only one or two positive cores (counter-intuitively this is irrespective of the number of cores), no core with more than 50% involvement and PSA density <0.15. The Epstein criteria were based on those biopsy criteria which predicted for the Stamey definition of clinically insignificant disease (<0.5 cc of Gleason 6 prostate cancer). This definition is stringent, and would exclude many patients with low risk disease who would otherwise be excellent candidates. Informed by the genetic characterization of Gleason pattern 3 and the clinical experience with Gleason 6, we believe that all ‘true’ Gleason 6 cancers (that is, without any occult Gleason 4 pattern)have an extremely low risk of metastasis. The major significance of higher volume disease is as a predictor of occult higher grade cancer. Higher volume disease may be manifested as extensive core involvement, a high PSA density, or a large lesion on MRI confirmed to be Gleason 6. In the absence of higher grade cancer, metastasis is exceedingly unlikely. Thus these patients require close scrutiny to preclude as much as possible co-existent higher grade disease, but do not necessarily require treatment in the absence of higher grade cancer. Biological progression to higher grade cancer may occur over time, and is higher volume disease is a predictor for this; but a possible risk of future grade progression should rarely drive current treatment decisions.
A high PSA density (PSA: prostate volume ratio) has been demonstrated in many studies to be a predictor for risk progression. A high PSA density in some surveillance candidates reflects PSA arising from a large occult cancer. Increased caution is warranted in these cases. In particular, this includes young men (age <55 years) who have extensive Gleason 6 cancer on biopsy. In these patients, uncertainty exists about. The risk of true tumour progression over time, as well as the risk of harboring occult high grade disease. It is reasonable to offer these men treatment. Where exactly to draw the line is a matter of clinical judgment.
Race plays a role. African Americans managed with surveillance have a higher rate of risk re-classification, and PSA failure when treated than Caucasian men (39). Black men who are surveillance candidates also have a higher rate of large anterior cancers than Caucasians (40). Japanese men younger than 60 have a lower rate of histological ‘autopsy’ cancer than Caucasian men. Thus the finding of low-grade prostate cancer in young Asian men is perhaps less likely to represent overdiagnosis. However, black and Asian patients diagnosed with low grade prostate cancer still include a majority of men who have little or no probability of a prostate cancer related-death during their remaining lives. Thus active surveillance is still an appealing option for those who have been appropriately risk-stratified. These higher risk patients are a group in whom improved imaging and biomarkers will likely have a major impact.
Further, the modification of the Gleason system in 2005 has resulted in a decrease in the number of newly diagnosed Gleason 6 compared to 7, and therefore a smaller proportion of prostate cancer patients classified as low risk and therefore fulfilling stringent criteria for surveillance. There is an increasing recognition that patients with Gleason 3+4=7, where the component of pattern 4 is small (<10%) have a very similar natural history to those with Gleason 3+3, perhaps reflecting the stage migration phenomenon. A recent pathology study showed that men with Gleason 3+ < 5% pattern 4 on biopsy had exactly the same distribution of cancer grades on radical prostatectomy pathology as those with Gleason 3 only (41).
Principles of management
The clinical management of men on AS has evolved. Currently most experienced clinicians use the following approach: Patients have PSA performed every 3 months for the first 2 years, and then every 6 months indefinitely (until infirmity). A confirmatory biopsy must be carried out within 6-12 months of the initial diagnostic biopsy on which cancer was identified. This confirmatory biopsy targets the areas of the prostate that have been shown to harbor significant cancer in patients who are initially diagnosed with Gleason 6: the anterior prostate, base, and apex. These are the areas are typically under-sampled on the initial diagnostic biopsy. If the confirmatory biopsy is either negative or confirms microfocal Gleason 3+3 disease, subsequent biopsies are performed every 3-5 years until the patient reaches age 80, or has a life expectancy <5 years because of co-morbidity. The frequency of biopsy varies widely between groups. Some have performed annual biopsies for many years. This approach has been valuable in establishing the likelihood of biological progression over time, but represents an overly large burden of biopsies. Multi-parametric MRI should be performed on those patients whose PSA kinetics suggest more aggressive disease (usually defined as a PSA DT <3 years), whose biopsy shows substantial volume increase, or who is upgraded to Gleason 3+4 and surveillance is still desired as a management option. Identification of an MRI target suspicious for high grade disease should warrant a targeted biopsy; or, if the lesion is large and unequivocal, intervention.
About one third of patients will be reclassified as higher risk and in most cases offered treatment. The proportion risk-reclassified will depend on the inclusion criteria used for eligibility for surveillance. An inclusive approach, offering surveillance to all patients with Gleason 6 and PSA <15, for example, will include more patients with occult high grade disease than a narrower approach, restricting surveillance to those who meet Epstein criteria. However, the more stringent eligibility denies the benefits of AS to many men with indolent disease who do not fit the Epstein criteria and thus are discouraged from choosing AS.
Most cases that are upgraded on the confirmatory or initial subsequent biopsy are upgraded based on re-sampling (about 25% of patients). Of those upgraded, more than 85% are upgraded to Gleason 3+4 only (42). In the Toronto cohort, the likelihood of upgrading increased by 1% per year from the time of the confirmatory biopsy (43). This is an estimate of the rate of spontaneous grade progression from Gleason 3 to Gleason 4.
The commonest cause of death in men on AS is cardiovascular disease. Death from prostate cancer is rare. In the most mature surveillance cohort (44), with a median follow-up of 8 years, the cumulative hazard ratio (or relative risk) of non-prostate-cancer death was 10 times that for prostate cancer. To date, the published literature on surveillance includes 14 prospective studies, encompassing about 5,000 men (44-58). Most of these studies have a duration of follow-up that is insufficient to identify an increased risk of prostate cancer mortality as a result of surveillance. For example, a Swedish study reported that the risk of prostate cancer mortality in patients managed by watchful waiting was low for many years, but tripled in patients who survived more than 15 years (59) (‘Watchful waiting’ meant no opportunity for selective delayed intervention, whereas about 30% of patients in the surveillance series have had radical treatment). In the Toronto experience, 70 patients have been followed for 14 years; 2.5% had late disease progression (with metastasis developing after 7 years), but there is no evidence of a sharp increase in mortality to date. Thus a critical question in this field is what the long term prostate cancer mortality will be beyond 15 years. It will be 5-7 years before the most mature cohorts have a substantial group of patients with more than 15 years of follow-up. Table 2 summarizes the results of the 10 prospective series. The key outcome measures include the proportion of patients treated, overall, and cause specific survival. Overall, about one third of patients are treated; most series have few or no prostate cancer deaths. In the Toronto cohort, 1.5% has died of prostate cancer; the actuarial 15-year prostate cancer mortality is 5%. In the Hopkins series, which was restricted patients to those with Epstein criteria, and treated all patients with volume progression beyond Epstein, the 15-year CSM was 0.5% (60). Few of the other publications have significant numbers of patients followed more than 10 years.
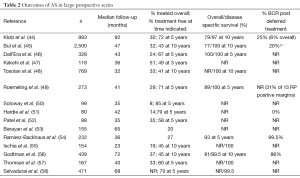
Full table
All groups have relied on systematic TRUS-guided biopsies performed serially, at varying intervals. This technique has significant limitations. TRUS guided biopsy tends to under sample the anterior prostate, apex, and antero-lateral horn. Thus all groups stress the importance of a confirmatory biopsy to target these areas. Since prostate cancer in most cases starts early and takes 10-20 years to reach clinical significance, the delay of 6-12 months in finding occult higher grade cancer is unlikely to alter curability significantly. MRI has an obvious increasing role in the management of AS patients. There are two potential benefits: reassurance that no higher risk disease is present in those with no visualized disease; and, in the subset harboring higher grade disease, earlier identification of this cancer. With respect to the former the negative predictive value is the key metric. This has been reported to be 97% for a group of about 300 surveillance candidates at MSKCC, and similar figure of 95-97% reported by several other groups (61,62). The PPV of an MRI abnormality with a PiRADS (Prostate Imaging Reporting and Data System) score of 4 or 5/5 had a 90% positive predictive value for high-grade cancer. This abnormality is characterized by a lesion with a positive T2-weighted image, with both restricted diffusion and enhanced contrast. These lesions should trigger a targeted biopsy. If confirmed by further studies, this reliability would permit a level of confidence in a negative MRI that would allow it to replace the biopsy. This would decrease the number of men requiring biopsies (a major unmet need) and facilitate early identification of clinically significant disease earlier.
PSA kinetics are currently used as a guide to identify patients at higher risk, but not to drive the decision to treat. Until multiparametric MRI became available, men on AS with poor PSA kinetics (doubling time <3 years) were offered treatment. In the PRIAS multi-institutional AS registry, 20% of men being treated had intervention based on a PSA doubling time <3 years (45). PSA kinetics is sensitive but lack specificity. For example, in a report of the 5 men dying of metastatic prostate cancer in the Toronto cohort, all had a PSA doubling time <2 years (63). In a study of PSA kinetics in a large surveillance cohort, false positive PSA triggers (doubling time <3 years, or PSA velocity >2 ng/year) occurred in 50% of stable untreated patients, none of whom went on to progress, require treatment, or die of prostate cancer, emphasizing the lack of sensitivity of a rapid rise in PSA (64). Vickers, in an overview of all of the studies of more than 200 patients examining the predictive value of PSA kinetics in localized prostate cancer, concluded that kinetics had no independent predictive value beyond the absolute value of PSA (65).
Active surveillance is an appealing approach for low risk patients, and an antidote to the widely recognized problem of overtreatment. Widespread adoption of surveillance would result in a reduction in the number NNT for each death avoided without the risk of increasing disease mortality. One hopes that a dispassionate re-assessment of PSA screening based on these improved metrics would lead to a re-consideration of the value of prostate cancer screening by organizations such as the USPSTF and the Canadian Task Force on the Public Health Exam. Ongoing improvements in diagnostic accuracy based on multiparametric MRI and genetic biomarkers should reduce the need for systematic biopsies, improve the early identification of occult higher risk disease, and enhance the ability to detect patients destined to have grade progression over time. A minimum standard currently is a confirmatory biopsy within 6–12 months. PSA should be performed every 6 months and subsequent biopsies every 3-5 years until the patient is no longer a candidate for definitive therapy. MRI is indicated for men with a grade or volume increase, or adverse PSA kinetics. Treatment should be offered for most patients with upgraded disease.
Focal therapy
A second, intermediate risk group, are also candidates for a tissue sparing approach. These are patients who either have small, unifocal Gleason 7 cancers, or larger Gleason 6 cancers confined to one lobe. Focal therapy is increasingly being advocated as a minimally invasive, less morbid alternative to conventional treatment. This is consistent with trends in surgical oncology in other tumour sites. Just as breast cancer, once treated routinely with radical mastectomy, is now widely managed with lumpectomy, tissue sparing treatment of small prostate cancers seems rational and appealing.
Focal therapy is based on the concept of the ‘index lesion’. Although most prostate cancers are multifocal, many patients have a single substantial lesion; the multifocality usually consists of small foci of low grade cancer scattered throughout the prostate. While the index lesion has not been demonstrated to invariably be the most aggressive lesion, clinical evidence suggests this is usually (although not invariably) the case.
Patient selection is critical to a successful outcome. The ideal patient has an unequivocal solitary lesion on MRI, confirmed as Gleason 7 on biopsy. Co-existent microfocal Gleason 6 disease elsewhere in the prostate is not a contra-indication. In the absence of the index lesion, the Gleason 6 microfoci would be managed conservatively. Selected small Gleason 8 cancers, in whom the rest of the prostate is normal on MRI, may also be managed in this fashion. The initial experience with focal therapy is impressive. A trifecta outcome, meaning continent, with normal erectile function and undetectable PSA, has been reported in 84%. The trifecta result with surgery and radiation are 40-50% (at centers of excellence) (66,67).
A key principle of patient selection is the use of an accurate technique to identify the presence or absence of aggressive prostate cancer. Uniquely in oncology, prostate cancer has been treated for many years without identifying the site of disease within the gland. Location was not critical if the entire prostate was removed or radiated, or if no treatment was offered. Advances in defining the location of disease now include MRI (68), image guided biopsy (69), and template prostate mapping (70). This has resulted in a reduction in under grading and risk assessment based on needle biopsy. Recent data on targeted biopsies have described a concordance rate of 95% with prostatectomy pathology, compared to 60% with systematic biopsies (69,71). This level of accuracy is paradigm shifting, in the sense that accurate assessment of extent of disease will permit treatment tailored to the location of this disease, rather than complete excision of the gland.
A diagnostic approach based on MRI with targeted biopsy will result in fewer men being biopsied and fewer cores per patient. The volume of disease on the core will increase dramatically as the needle is directed towards the lesion center. The traditional parameters of cancer core length, proportion of involvement, and risk will have to be re-calibrated based on targeted biopsies. For example, a Gleason 6 0.5 cc lesion, corresponding to Stamey’s volume threshold for significance, if hit directly, could result in a cancer core length of 10-11 mm, or 75% of a 14-mm core. Based on having clinically insignificant disease by the most stringent criteria, such a patient should be managed with surveillance, but may be dissuaded from doing so by conventional risk stratification systems.
A targeted approach to biopsy based on imaging will also result in fewer men found to have clinically insignificant prostate cancer. Although such men may avoid the side effects of therapy, they still are subject to the anxiety attendant upon a cancer diagnosis, the ‘survivor’ label, and repeated diagnostic studies. Reducing overdiagnosis, even if overtreatment is avoided, would be a major public health benefit.
In applying focal therapy, 3 different imaging requirements are present, each with different demands. Imaging is required (I) for patient selection, ie men with low-intermediate risk prostate cancer (Gleason ≤4+3, PSA <20 ng/mL, and ≤T2), with a target lesion confined to one lobe of the prostate; (II) for real time treatment guidance of the therapy to the targeted lesion; and (III) follow-up to confirm no residual or recurrent tumor. Focal therapy emerged as a plausible approach only with the availability of MP-MRI, beginning around 2009, which for the first time made accurate imaging of prostate cancer feasible.
The published series have modest numbers and short follow-up. A systematic review summarized this data (67). The results, summarized in Table 3, are as follows. Focal therapy is safe in the short term. The GU and rectal morbidity is low. No prostate cancer deaths or metastases have been reported, likely reflecting the absence of long term follow-up. Oncological outcomes are favorable, with freedom from disease recurrence of 80-85% (using a variety of definitions of recurrence) (Table 4). Re-treatment is required in about 10%. A change in treatment was applied in 5%. Longer follow-up is required to validate these findings.
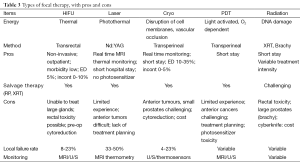
Full table
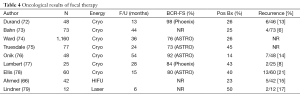
Full table
Technique of focal therapy
A variety of techniques have been described, all involving the use of directed energy and image guidance. These include high intensity focused ultrasound (HIFU), MR guided ultrasound, laser ablation, cryosurgical ablation, focal photodynamic therapy, electroporation, various forms of radiation. Ultimately, which of these therapies becomes widely used will be a reflection of precision of treatment, morbidity, cost, and availability and convenience. The principles and methods used with these directed energies have been described previously. The experience with these technologies used for focal therapy is summarized in the table below, in chronological order.
Most of the focal therapy data lacks robust endpoints. In most published studies, follow-up biopsies were usually not systematic, and in most studies the majority of patients were not biopsied. This is a potential source of bias, in that PSA and MRI may misidentify as responders some patients with residual disease. In patients having a biopsy, the rate of positive biopsies ranged from 14% to 50% (Table 2). Further, most authors only biopsied the treated area. Biopsies of the untreated area were selective based on mpMRI.
It is predictable that a non-morbid, minimally invasive therapy for a slow growing disease will produce excellent short term clinical results. The key question is that of the long term durability of this therapy with respect to local recurrence and metastasis. This is unknown. The FDA, in a recent attempt to develop surrogate end points to evaluate focal therapy, concluded that neither PSA, biopsy showing absence of upgrading, or MRI changes, fulfilled the criteria for a valid endpoint for confirming the long term benefit of focal treatment. They did not identify a putative marker that would fulfill these criteria. Recently, the FDA has changed its requirements for approval of minimally invasive therapies. These devices must now demonstrate that they are effective and safe for tissue ablation, rather than demonstrating that the outcome of these treatments is equivalent to other therapies.
Although the idea of focal treatment is simple, the application has many nuances and unresolved issues. Challenges include accurate visualization and characterization of significant cancer foci, establishing rational criteria for patient selection, precise localization of therapy matched to the targeted lesion and accurate and precise direction of the ablative energy into the area to be targeted, and post treatment surveillance strategies. Most importantly, establishing the effectiveness of focal therapy in altering the natural history of low-intermediate risk prostate cancer will require large series with long follow-up. This will take several decades and perhaps more.
Conclusions
Most, if not all Gleason 6 cancers lack metastatic potential. Conservative management in these cases, which represent about 45% of newly diagnosed prostate cancers in a screened population, is warranted as an initial strategy. The objective of management is early identification of occult higher grade cancer and long-term follow-up to identify the minority of patients who exhibit grade progression over time. Several large cohorts with follow-up of 10-15 years have confirmed the safety of this approach. In younger men with extensive Gleason 6 cancer, or those whose imaging identifies an index lesion, treatment may be warranted. Minimally-invasive therapies, including focal therapy based on precise mp-MRI targeting, have an emerging role in this context. With widespread adoption of this approach, the number NNT radically in a screened population for each death avoided will fall substantially. This will enhance the value and appeal of early detection for prostate cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-34. [PubMed]
- Canadian Task Force on Preventive Health Care. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ 2014;186:1225-34. [PubMed]
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050-8. [PubMed]
- Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst 2005;97:1248-53. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Ross AE, Marchionni L, Vuica-Ross M, et al. Gene expression pathways of high grade localized prostate cancer. Prostate 2011;71:1568-77. [PubMed]
- Skacel M, Ormsby AH, Pettay JD, et al. Aneusomy of chromosomes 7, 8, and 17 and amplification of HER-2/neu and epidermal growth factor receptor in Gleason score 7 prostate carcinoma: a differential fluorescent in situ hybridization study of Gleason pattern 3 and 4 using tissue microarray. Hum Pathol 2001;32:1392-7. [PubMed]
- Padar A, Sathyanarayana UG, Suzuki M, et al. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin Cancer Res 2003;9:4730-4. [PubMed]
- Susaki E, Nakayama KI. Multiple mechanisms for p27(Kip1) translocation and degradation. Cell Cycle 2007;6:3015-20. [PubMed]
- True L, Coleman I, Hawley S, et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci U S A 2006;103:10991-6. [PubMed]
- Guo Y, Sklar GN, Borkowski A, et al. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 1997;3:2269-74. [PubMed]
- Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet 2007;39:41-51. [PubMed]
- Fleischmann A, Huland H, Mirlacher M, et al. Prognostic relevance of Bcl-2 overexpression in surgically treated prostate cancer is not caused by increased copy number or translocation of the gene. Prostate 2012;72:991-7. [PubMed]
- Hendriksen PJ, Dits NF, Kokame K, et al. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res 2006;66:5012-20. [PubMed]
- Serrano M. Cancer regression by senescence. N Engl J Med 2007;356:1996-7. [PubMed]
- Bismar TA, Dolph M, Teng LH, et al. ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur J Cancer 2012;48:538-46. [PubMed]
- Furusato B, Gao CL, Ravindranath L, et al. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod Pathol 2008;21:67-75. [PubMed]
- Sowalsky AG, Ye H, Bubley GJ, et al. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res 2013;73:1050-5. [PubMed]
- Wang J, Cai Y, Ren C, et al. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res 2006;66:8347-51. [PubMed]
- West AF, O'Donnell M, Charlton RG, et al. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer 2001;85:576-83. [PubMed]
- Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011;185:869-75. [PubMed]
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) £ 6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012;36:1346-52. [PubMed]
- Ahmed HU, Arya M, Freeman A, et al. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol 2012;13:e509-17. [PubMed]
- Font-Tello A, Juanpere N, de Muga S, et al. Association of ERG and TMPRSS2-ERG with grade, stage, and prognosis of prostate cancer is dependent on their expression levels. Prostate 2015. [Epub ahead of print]. [PubMed]
- Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013;123:4918-22. [PubMed]
- Barbieri CE, Demichelis F, Rubin MA. The lethal clone in prostate cancer: redefining the index. Eur Urol 2014;66:395-7. [PubMed]
- Haffner MC, De Marzo AM, Yegnasubramanian S, et al. Diagnostic challenges of clonal heterogeneity in prostate cancer. J Clin Oncol 2015;33:e38-40. [PubMed]
- Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012;106:1095-9. [PubMed]
- Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 2013;14:690. [PubMed]
- Klein EA, Yousefi K, Haddad Z, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur Urol 2015;67:778-86. [PubMed]
- Robinson K, Creed J, Reguly B, et al. Accurate prediction of repeat prostate biopsy outcomes by a mitochondrial DNA deletion assay. Prostate Cancer Prostatic Dis 2010;13:126-31. [PubMed]
- Cullen J, Rosner IL, Brand TC, et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur Urol 2015;68:123-31. [PubMed]
- Fascelli M, George AK, Frye T, et al. The role of MRI in active surveillance for prostate cancer. Curr Urol Rep 2015;16:42. [PubMed]
- Duffield AS, Lee TK, Miyamoto H, et al. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J Urol 2009;182:2274-8. [PubMed]
- Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo 1994;8:439-43. [PubMed]
- Bratt O, Folkvaljon Y, Loeb S, et al. Upper limit of cancer extent on biopsy defining very low-risk prostate cancer. BJU Int 2014. [Epub ahead of print]. [PubMed]
- Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw 2014;12:686-718. [PubMed]
- Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol 2013;31:2991-7. [PubMed]
- Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol 2014;191:60-7. [PubMed]
- Huang CC, Kong MX, Zhou M, et al. Gleason score 3+4=7 prostate cancer with minimal quantity of gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am J Surg Pathol 2014;38:1096-101. [PubMed]
- Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 2011;29:2795-800. [PubMed]
- Jain S, Loblaw A, Vesprini D, et al. Gleason Upgrading with Time in a Large Prostate Cancer Active Surveillance Cohort. J Urol 2015. [Epub ahead of print]. [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [PubMed]
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [PubMed]
- Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008;112:2664-70. [PubMed]
- Kakehi Y, Kamoto T, Shiraishi T, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol 2008;38:122-8. [PubMed]
- Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 2011;29:2185-90. [PubMed]
- Roemeling S, Roobol MJ, de Vries SH, et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. Eur Urol 2007;51:1244-50; discussion 1251. [PubMed]
- Soloway MS, Soloway CT, Williams S, et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int 2008;101:165-9. [PubMed]
- Hardie C, Parker C, Norman A, et al. Early outcomes of active surveillance for localized prostate cancer. BJU Int 2005;95:956-60. [PubMed]
- Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol 2004;171:1520-4. [PubMed]
- Barayan GA, Brimo F, Bégin LR, et al. Factors influencing disease progression of prostate cancer under active surveillance: a McGill University Health Center cohort. BJU Int 2014;114:E99-E104. [PubMed]
- Ramirez-Backhaus M, Iborra I, Gomez-Ferrer A, et al. Prostatectomy pathology findings in an active surveillance population. Arch Esp Urol 2014;67:431-9. [PubMed]
- Ischia JJ, Pang CY, Tay YK, et al. Active surveillance for prostate cancer: an Australian experience. BJU Int 2012;109 Suppl 3:40-3. [PubMed]
- Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial. Eur Urol 2013;63:101-7. [PubMed]
- Thomsen FB, Røder MA, Hvarness H, et al. Active surveillance can reduce overtreatment in patients with low-risk prostate cancer. Dan Med J 2013;60:A4575. [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [PubMed]
- Popiolek M, Rider JR, Andrén O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol 2013;63:428-35. [PubMed]
- Tosoian J, Mamawala M, Epstein J, et al. PD6-04: A Prospective, Longitudinal Active Surveillance Program for Favorable-Risk Prostate Cancer: Long Term Outcomes. New Orleans: AUA, 2015.
- Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 2012;188:1732-8. [PubMed]
- Okoro C, George AK, Siddiqui MM, et al. MRI/TRUS Fusion Prostate Biopsy Significantly Outperforms Systematic 12-Core Biopsy for Prediction of Total MRI Tumor Volume in Active Surveillance Patients. J Endourol 2015. [Epub ahead of print]. [PubMed]
- Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol 2010;184:131-5. [PubMed]
- Loblaw A, Zhang L, Lam A, et al. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J Urol 2010;184:1942-6. [PubMed]
- Vickers AJ, Savage C, O'Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398-403. [PubMed]
- Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622-32. [PubMed]
- Valerio M, Ahmed HU, Emberton M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 2014;66:732-51. [PubMed]
- Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. [PubMed]
- Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63:125-40. [PubMed]
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology 2007;70:27-35. [PubMed]
- Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate 2013;73:778-87. [PubMed]
- Durand M, Barret E, Galiano M, et al. Focal cryoablation: a treatment option for unilateral low-risk prostate cancer. BJU Int 2014;113:56-64. [PubMed]
- Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 2012;62:55-63. [PubMed]
- Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int 2012;109:1648-54. [PubMed]
- Truesdale MD, Cheetham PJ, Hruby GW, et al. An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: recommendations for follow-up. Cancer J 2010;16:544-9. [PubMed]
- Onik G, Vaughan D, Lotenfoe R, et al. The "male lumpectomy": focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol 2008;26:500-5. [PubMed]
- Lambert EH, Bolte K, Masson P, et al. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology 2007;69:1117-20. [PubMed]
- Ellis DS, Manny TB Jr, Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology 2007;70:9-15. [PubMed]
- Lindner U, Weersink RA, Haider MA, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol 2009;182:1371-7. [PubMed]


