Mast cells, estrogens, and cryptorchidism: A histological based review
Introduction
An undescended testis occurs in approximately 3.7% of boys at birth and 1.1% will still have undescended testes at 1 year of age (1). If untreated, cryptorchidism leads to age dependent decreases in germ cell number (GCN) and testicular fibrosis (2,3). The pathophysiology of this process and its long-term effects on infertility are unclear. Increases in mast cell numbers have been linked to peritubular fibrosis in adults presenting with infertility (4,5). Mast cells are activated by estrogens and involved in interstitial remodeling in the female reproductive cycle but their role in the developing testis is unknown (6). There are no studies of mast cells in cryptorchid testis in humans but several animal models have investigated mast cells and their response to estrogens. In this review we will examine the possible links between estrogens, mast cells, and testicular fibrosis in cryptorchidism, focusing on histological studies.
Cryptorchidism and fibrosis
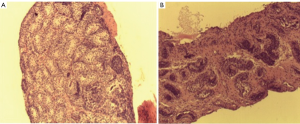
In 1982 Miniberg et al. performed electron microscopy on testicular biopsy specimens from cryptorchid testes taken at the time of orchidopexy. They found that the interstitial testicular architecture became increasing more disorganized the older a child was at the time of surgery. They also noted an increase in fibroblast number and collagen deposition and a decrease in leydig cell numbers. These findings became increasingly more prominent after one year of age. Suskind et al. retrospectively reviewed testicular biopsy specimens from 86 subjects with cryptorchidism (3). Mean seminiferous tubular diameter, germ cell number and degree of fibrosis were assessed. Tubular diameter and germ cell number were significantly higher in boys less than 1yr of age. Fibrosis was worse in older boys and was associated with smaller tubular diameter. Park et al. prospectively performed testis biopsies at the time of orchidopexy for unilateral cryptorchidism. They found that in patients younger that 1 yr, fertility index and germ cell number were significantly higher than those older than 1 yr (2). The interstitial fibrosis index (a measure of the percent of fibrosis of testis tissue) was significantly lower in boys less than two compared older boys. It is clear that timing of orchidopexy predicts the degree of fibrosis (and loss of functional seminiferous tubules) but the etiology of this fibrosis is unclear. Figure 1 demonstrates varying amounts of fibrosis in testicular biopsies of boys undergoing orchidopexy at different ages.
Mast cells and infertility
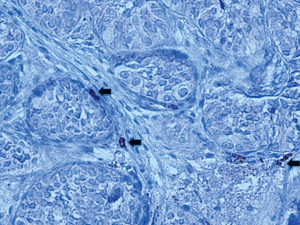
Mast cells activate fibroblasts and promote collagen synthesis by producing and releasing proteolyic enzymes (ie. trypsin) and other substances associated with inflammation and fibrosis. Mast cells are found in small numbers around blood vessels in the interstitium of human testes with normal spermatogenesis (7). However, they are increased in number in testes of infertile men and those with testicular atrophy (5,8,9). Increased numbers of mast cells in infertile males are seen both in the interstitium and around seminiferous tubules (4,8,10). Increases in peritubular mast cells are associated with peritubular fibrosis (4,5). Mast cell numbers correlate with defective spermatogenesis and this effect is most pronounced in testicular biopsies showing maturation arrest and Sertoli Cell only patterns (7,8,11). There is a clear correlation between MC number and fibrosis in the testes of infertile adults. Currently, there are no studies reporting mast cell numbers in cryptorchid or developing testis in humans. We examined biopsies of undescended testes with trypsin staining and found mast cells in the interstitium and peritubular locations (unpublished study). Figure 2 shows trypsin staining of mast cells in a testis biopsy taken at the time of orchidopexy.
Mast cells and fibrosis
Mast cells have been found to promote fibrosis by stimulating fibroblast proliferation and collagen deposition. In vitro studies from both human and animal models have shown that the mast cell proteases, chymase and trypsin, induce fibroblast cell proliferation (12,13). Ohtsuka et al. found that human mast cell sonicates increased fibroblast collagen synthesis 9 fold and protein synthesis 11 fold (14). In this preparation, mast cell products also exhibited a cytotoxic effect on endothelial cells that was reversed with a trypsin inhibiter. Garbazenko et al. also found that fibroblast proliferation and collagen synthesis increased significantly in a dose dependent fashion when exposed to mast cell sonicates (15). They saw a similar effect when fibroblasts were exposed to the pre-formed mast cell mediators histamine and tryptase. Available evidence demonstrates that mast cell products activate fibroblasts and collagen synthesis in tissue culture models.
Another way in which mast cells may influence fibrosis is through activation of matrix metalloproteinases (MMPs). MMPs are a family of enzymes (proteinases) involved in the remodeling of the extracellular matrix and in normal development (16) MMP 2 and 9 are 2 proteases that have the ability to degrade collagen (17). Pro-MMP 2 and 9 (unactivated MMPs) were shown to be mast cell and chymase dependant in knock-out mice that lacked the homologous enzyme to human chymase (18). Kimata et al. used a human cultured mast cell model to show that MMP-9 is produced de-novo through the PKC-MEK-ERK pathway in mast cells (19). MMPs have also been found in developing human testes. Robinson et al. examined gonadal tissue from human fetuses after termination of pregnancy and found that MMP-2 and 9 were expressed (20). MMPs are involved in a variety of cellular functions including growth and differentiation.
Estrogens and mast cell activation
Human mast cell lines have been found to naturally express α estrogen receptors (αER) (21). Zaitsu, et al., demonstrated that estradiol (E2) caused degranulation of mast cells resulting in the release of β-Hexaminidase and Leukotrienes (mediators of inflammation). E2 also augmented the IgE mediated allergic response in these in-vitro experiments. When E2 was added to mast cells derived from Estrogen Receptor-α knockout mice no degranulation occurred. Furthermore, the addition of Tamoxifen (a competitive inhibitor of Estrogen Receptors) inhibited the E2 driven release of degranulation of mast cells. Human mast cells normally express αERs and they respond to physiologic levels of estrogen.
Mast cell migration and activation has been studied extensively in the female reproductive cycle. The proteolytic properties of mast cells have implicated them as possible mediators in extracellular matrix degradation and trophoblastic invasion. Jenson, et al., evaluated the role of estrogen in mast cell migration and activation in response to uterine cells. They showed that human mast cells migrated toward uterine cells when exposed to physiologic levels of estradiol and upregulated certain chemokine receptors (CCR3 and CCR5) (6) that are involved in chemotaxis and migration (22). They also demonstrated upregulation of human trypsin expression and increased mast cell degranulation with E2 exposure. Increased trypsin expression along with the demonstration of degranulation suggests that these are mature and activated mast cells. These studies show a strong cause and effect relationship between estrogen exposure and mast cell activation in human mast cell lines.
The effects of estrogen and estrogen receptor expression in developing testes
Mizuno, et al. used a cryptorchid rat model derived from pregnant Sprague-Dawley rats exposed to flutamide to investigate estrogen receptor-α and β expression (23). In this model real time PCR and immunohistochemical studies demonstrated that the estrogen receptor-α protein expression was strong in spermatid germ cells in descended testes and in contrast, there was no expression in germ cells of cryptorchid testes at 7 weeks. After orchiopexy estrogen receptor-α was detected in spermatids of cryptorchid testes. Interestingly, estrogen receptor-α was strongly expressed in Leydig cells of cryptorchid testes and only weakly expressed in those of controls. Estrogen receptor-β expression was no different between groups. The authors concluded that increased estrogen receptor-α expression in cryptorchid Leydig cells correlated with increased intratesticular estrogen. This is based on the premise that estrogens upregulate estrogen receptor expression. In these cryptorchid testes spermatogenic arrest occurred at the spermatid differentiation stage and estrogen receptor-α expression seemed to correlate with overall germ cell number and degree of impairment of spermatogenesis.
Bilinska et al. studied aromatase expression in the testes of two different strains of mice with immunohistochemical and steroid analysis (24). There was moderate to strong staining of aromatase in Leydig and germ cells, especially spermatids and spermatocytes of both control and cryptorchid testes. Estrogen receptor-α expression was confined to Leydig cells in both groups. Analysis of homogenized tissue showed that testosterone levels were significantly lower and estradiol levels significantly higher in cryptorchid testes as compared to controls. In a transgenic mouse model, aromatase overexpression correlated with Leydig cell hyperplasia and hypertrophy (25). There was also severe disruption of spermatogenesis with almost no germ cells at 15 months of age in mice in the overexpression group. Intratesticular E2 levels were significantly elevated and FSH levels decreased compared to wild type controls.
In addition, there was an increase in fibrosis, TNF-α staining, and an increased numbers of macrophages and mast cells in testes with aromatase overexpression in the transgenic mouse model (24). These studies by Mizuna and Bilinska together demonstrated that estrogen levels are increased in cryptorchid testes and E2 targets the estrogen receptor-α on Leydig cells and germ cells. This results in Leydig cell hyperplasia and can have deleterious effects on spermatogenesis. E2 may also contribute to local inflammatory responses in the testicular interstitium.
Estrogens and mast cells in testicular animal models
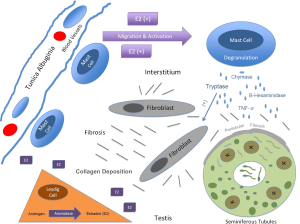
An increase in mast cell number has been shown to result from estrogen stimulation in the testis in several animal models. Gaytan et al. injected neonatal rats with testosterone or estradiol at birth and examined mast cell numbers after 6 weeks. In control rats mast cells were exclusively located under the tunica albuginia around blood vessels and they exhibited normal spermatogenesis. The findings were the same in the testosterone treated group. However in the E2 treated group mast cells were found in the interstitium in increased numbers and spermatogenesis was impaired. In a frog model Minucci and colleages examined mast cell number after 2 weeks of testosterone or estrogen injection using light microscopy and electron microscopy (26). E2 increased mast cell numbers significantly and the mast cells were noted to contain mature granules. This effect was reversed with tamoxifen. It is clear that mast cells are regulated by estrogen and that this interaction has an effect on the developing testes in animals. There have been no studies in humans examining mast cell numbers in response to estrogen exposure in the developing testes. Figure 3 depicts a proposed model for the interaction between mast cells, estrogens, and germ cells in cryptorchid testes.
Conclusions
Mast cells are intricately involved in inflammation and fibrosis in a variety of organ systems. Their secretory products (tryptase, chymase, etc.) have mitogenic effects on fibroblasts and promote collagen deposition. Mast cell activation and migration are under the influence of estrogens and this interaction has been demonstrated in testes in several animal models. Mast cell numbers have a positive correlation with testicular fibrosis and are associated with deceased spermatogenesis in adults. To date, their involvement in the pathological fibrosis seen in cryptorchidism is unknown. However the above evidence suggests that cryptorchid testes have an increase in intratesticular estrogen, which promotes mast cell migration and proliferation. Mast cell secretory products may contribute to the fibrosis of undescended testes. The role of mast cells in the pathophysiology of cryptorchidism and their effect on future fertility warrants further study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Berkowitz GS, Lapinski RH, Dolgin SE, et al. Prevalence and natural history of cryptorchidism. Pediatrics 1993;92:44-9. [PubMed]
- Park KH, Lee JH, Han JJ, et al. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int J Urol 2007;14:616-21. [PubMed]
- Suskind A, Hayner-Buchan A, Feustel PJ, et al. Fibrosis correlates with detailed histological analysis of human undescended testes. BJU Int 2008;101:1441-5. [PubMed]
- Apa DD, Cayan S, Polat A, et al. Mast cells and fibrosis on testicular biopsies in male infertility. Arch Androl 2002;48:337-44. [PubMed]
- Yamanaka K, Fujisawa M, Tanaka H, et al. Significance of human testicular mast cells and their subtypes in male infertility. Hum Reprod 2000;15:1543-7. [PubMed]
- Jensen F, Woudwyk M, Teles A, et al. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One 2010;5:e14409. [PubMed]
- Hussein M R, Abou-Deif ES, Bedaiwy MA, et al. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil Steril 2005;83:1447-53. [PubMed]
- Roaiah MM, Khatab H, Mostafa T. Mast cells in testicular biopsies of azoospermic men. Andrologia 2007;39:185-9. [PubMed]
- Jezek D, Banek L, Hittmair A, et al. Mast cells in testicular biopsies of infertile men with ‘mixed atrophy’ of seminiferous tubules. Andrologia 1999;31:203-10. [PubMed]
- Meineke V, Frungieri MB, Jessberger B, et al. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 2000;74:239-44. [PubMed]
- Sezer C, Koksal IT, Usta MF, et al. Relationship between mast cell and iNOS expression in testicular tissue associated with infertility. Arch Androl 2005;51:149-58. [PubMed]
- Algermissen B, Hermes B, Feldmann-Boeddeker I, et al. Mast cell chymase and tryptase during tissue turnover: analysis on in vitro mitogenesis of fibroblasts and keratinocytes and alterations in cutaneous scars. Exp Dermatol 1999;8:193-8. [PubMed]
- Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest 1991;88:493-9. [PubMed]
- Ohtsuka T. Different interaction of mast cells with human endothelial cells and fibroblasts. Eur J Dermatol 2000;10:115-21. [PubMed]
- Garbuzenko E, Nagler A, Pickholtz D, et al. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 2002;32:237-46. [PubMed]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 2003;200:448-64. [PubMed]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 2000;14:2123-33. [PubMed]
- Tchougounova E, Lundequist A, Fajardo I, et al. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem 2005;280:9291-6. [PubMed]
- Kimata M, Ishizaki M, Tanaka H, et al. Production of matrix metalloproteinases in human cultured mast cells: involvement of protein kinase C-mitogen activated protein kinase kinase-extracellular signal-regulated kinase pathway. Allergol Int 2006;55:67-76. [PubMed]
- Robinson LL, Sznajder NA, Riley SC, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human fetal testis and ovary. Mol Hum Reprod 2001;7:641-8. [PubMed]
- Zaitsu M, Narita S, Lambert KC, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol 2007;44:1977-85. [PubMed]
- Ochi H, Hirani WM, Yuan Q, et al. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med 1999;190:267-80. [PubMed]
- Mizuno K, Kojima Y, Kurokawa S, et al. Altered expression and localization of estrogen receptors alpha and beta in the testes of a cryptorchid rat model. Urology 2011;77:251.e1-6.
- Bilińska B, Kotula-Balak M, Gancarczyk M, et al. Androgen aromatization in cryptorchid mouse testis. Acta Histochem 2003;105:57-65. [PubMed]
- Li X, Strauss L, Kaatrasalo A, et al. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology 2006;147:1271-7. [PubMed]
- Minucci S, Di Matteo L, Chieffi P, et al. 17 beta-estradiol effects on mast cell number and spermatogonial mitotic index in the testis of the frog, Rana esculenta. J Exp Zool 1997;278:93. [PubMed]