Extension of the therapeutic spectrum in castration-resistant prostate cancer: Osteoclast inhibition with denosumab
Denosumab is a human monoclonal antibody (IgG2) that binds to RANK ligand (RANKL). It reduces osteoclasts and thereby decreases bone resorption and cancer-induced bone destruction.
A randomized, double-blind, placebo-controlled phase-3 study evaluated the effect of denosumab on bone metastasis-free survival in men with castration-resistant prostate cancer (CRPC) (1). They were at high risk for the development of bone metastases. This was defined as a prostate-specific antigen (PSA) value of ≥8 ng/mL and/or a PSA doubling time of ≤10 months. Patients were randomized 1:1 to receive denosumab 120 mg subcutaneously (SC) every 4 weeks (Q4W; n=716) or placebo SC Q4W (n=716). The main exclusion criteria included evidence of radiographically detectable bone metastases, evidence of viszeral metastases (except lymph nodes), intravenous bisphosphonate use, and history or evidence of osteonecrosis of the jaw. Patients were recommended to supplement calcium ≥500 mg and vitamin D≥400 IU. The primary endpoint of the study was bone metastasis-free survival; this was determined by the time from randomization to the first occurrence of bone metastasis or death. Secondary endpoints were time to first bone metastasis and overall survival. Patients were exposed to denosumab for a median of 19 months and to placebo for a median of 18 months; patients remained on study for a median of 20.2 and 19.0 months, respectively.
The median bone metastasis-free survival was significantly increased by 4.2 months in the denosumab arm compared to the placebo arm [(29.5 versus 25.2 months; hazard ratio (HR): 0.85; P=0.028]. The median time to first bone metastasis was also significantly increased in the denosumab arm compared to the placebo arm (33.2 versus 29.5 months; HR: 0.84; P=0.032). Symptomatic bone metastases were reported in 69 (10%) and 96 (13%) patients in the denosumab and placebo arms, respectively (P=0.03), and the time to a symptomatic bone metastasis was significantly delayed in the denosumab arm compared to the placebo arm (HR=0.67; P=0.01). No differences in OS (HR=1.01; P=0.91) or progression-free survival (HR=0.89; P=0.09) were reported between the two arms.
Results are summarized in Table 1.
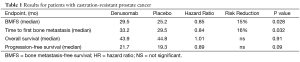
Full table
Adverse events were reported similiarly in both arms, except for osteonecrosis of the jaw: 94% of patients in the denosumab arm and 93% of patients in the placebo arm. The results are summarized in Table 2.
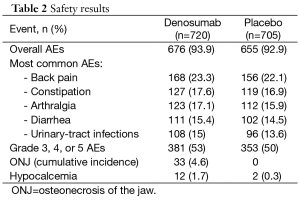
Full table
This study demonstrates that in patients with CRPC a bone-targeted therapy can delay the time to bone metastasis. Already in 1889 Stephen Paget proposed the seed and soil hypothesis between tumour cells and host microenvironment to explain the spread to different anatomic sites of different cancers. A vicious cycle of complex interactions between prostate cancer cells and bone microenvironment is suggested. Therefore the study highlights the important role of bone microenvironment and RANKL signalling in the development of bone metastases in men with CRPC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone metastasis-free survival in men with castration-resistant prostate cancer: results of a global phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39-46. [PubMed]


