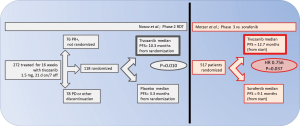
Milestones for development of tivozanib for kidney cancer therapy
In the May 10, 2012 issue of the Journal of Clinical Oncology, Nosov and colleagues report on a company-sponsored (AVEO), phase II randomized discontinuation trial (RDT) of tivozanib therapy for metastatic kidney cancer (1). This trial is part of a development of tivozanib seeking to expand the growing list of active medical treatment options blocking the vascular endothelial growth factor (VEGF) pathway, besides drugs with mammalian target of rapamycin (mTOR) pathway inhibition, and treatments using immune mechanisms. Among the VEGF pathway approaches, small molecule tyrosine kinase (TKI) inhibitors already approved for use in metastatic renal cell carcinoma include sunitinib, sorafenib, pazopanib, and most recently axitinib; bevacizumab is an antibody that binds plasma VEGF, so that the VEGF receptor remains without this ligand. Tivozanib is distinguished from the other small molecule VEGFR TKI by a more potent inhibition of VEGF receptors 1, 2 and 3 (half-maximal inhibition at 0.24 nmol/L or lower), and a long half-life (reported here at 87.0±27.9 hours) (1). The high potency is reflected in the relatively lower daily dose, 1.5 mg daily, versus daily doses of 10-800 mg for the others. Additionally, the relative potency for VEGFR1, VEGFR2 and VEGFR3 versus the inhibition of kinases that are not VEGF receptors is over 10 fold, which is higher than for other members of the group. This feature may decrease off-target inhibition, impacting side effects or therapeutic effects (1,2).
The randomized discontinuation trial (RDT) format for cancer therapy evaluations was introduced to kidney cancer therapeutics during the development of sorafenib (3). The method allocates those with major responses to continue on treatment, and those with progression or intolerance to stop treatment; the middle group, with nominally stable disease at a landmark time point, are randomized to continue on treatment or to stop, through the next evaluation time point. The strength of the method is to address the known heterogeneity of rates of progression as is typically observed in the population with metastatic kidney cancer, and to emphasize progression-free survival (PFS), among those initially achieving a major response. In the sorafenib RDT trial 65 patients who had stable disease at the landmark were randomized to continue sorafenib or receive placebo, and median PFS of 24 vs. 6 weeks (P=0.0087) favored sorafenib (3). The subsequent randomized pivotal phase III trial of sorafenib vs. placebo confirmed superiority of sorafenib for PFS endpoint, (5.5 vs. 2.8 months, P<0.01), and overall survival improvement was observed as well (19.3 vs. 15.9 months, P=0.02; this P-value was not significant by the prespecified boundary rules) (4).
In the report here, 306 worldwide patients were assessed for eligibility over about 10 months in 2007-2008, and 272 were treated. By the 16 week time point, 78 of had tumor size decreases of >25%, qualifying for continuation on open-label drug, and 76 were discontinued from the trial, for progressive disease or other reasons. The primary endpoint of overall response rate at the 16 week point was 18% (95% CI: 14-23%), and counting responses qualifying after further therapy, 24% (95% CI: 19-30%). Among 118 patients allocated to double-blind randomization for 12 weeks of treatment, 61 were assigned to tivozanib and 57 to placebo. After progression during the 12 weeks double-blind treatment, 24 of the placebo group were switched to open-label tivozanib. The median PFS (counted from the point of random assignment) significantly favored continuation of tivozanib, 10.3 vs. 3.3 months, P=0.010). The majority of patients enrolled had clear cell histology and had undergone nephrectomy, approximately half were treatment naïve. A subset with greater benefit was those with clear cell histology and prior nephrectomy (ORR 32%, median PFS 14.8 months) (1). Most common reported adverse events reported were hypertension and dysphonia, which may be considered “on-target” related with respect VEGFR-TKI inhibition (1,2).
In June 2012 at the ASCO annual meeting in Chicago, the pivotal trial of tivozanib vs. sorafenib [NCT01030783], a direct comparison between two active agents, was presented by Motzer and colleagues. The meeting report describes the 517 patient trial in patients with metastatic kidney cancer who had undergone nephrectomy, who were either treatment naïve or had received one prior systemic therapy excluding VEGF or mTOR directed therapy. Patients were randomized to receive tivozanib 1.5 mg daily for three weeks followed by 1 week off therapy [the same as in (1)] or sorafenib given continuously at 400 mg twice daily. Results show the positive finding of a median PFS in the tivozanib arm of 12.7 months compared to 9.1 months in sorafenib arm (HR 0.756, 95% CI: 0.580-0.985, P=0.037). In the treatment-naïve subset, the results were similar (5). Overall survival data were not mature, but contemporary availability of many second-line approaches can be anticipated to attenuate the power to observe a difference.
Although the relative frequency of side effects was not the primary endpoint of the trial, they are of interest in this direct comparison. This serves to address the hypothesis that tivozanib and axitinib have more relative specificity for the VEGFR TKI, thus being different from sorafenib, sunitinib or pazopanib, which also have significant inhibition of the activity of proteins such as B-raf, C-kit, or FGFR1. Hypertension, dysphonia and back pain were seen at higher frequency with tivozanib, while diarrhea, palmar-plantar erythrodysesthesia and alopecia were seen more frequently with sorafenib.
With over 500 clear cell kidney cancer patients treated with tivozanib, observed major responses, statistically significant improvement of PFS in the RDT format trial, and statistically significant improved PFS in direct comparison to sorafenib, there is little doubt that tivozanib is a new drug that is practical for use and can impact the disease course for a significant fraction of kidney cancer patients (Figure 1). The challenges remaining for drug development and market introduction in an indication of metastatic kidney cancer will be to see to what extent differences of side effects, cost, convenience and the capacity for synergistic combination may matter, potentially to make tivozanib a better choice for some patients.
Acknowledgements
Funding: Dr. Fishman has received research funding from AVEO through participation in NCT00563147 tivozanib + temsirolimus combination, citation #28 within (1).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nosov DA, Esteves B, Lipatov ON, et al. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol 2012;30:1678-85. [PubMed]
- Gupta S, Fishman M. Progress and contrasts of the development of tivozanib for therapy of kidney cancer. Expert Opin Pharmacother 2011;12:2915-22. [PubMed]
- Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:2505-12. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Robert John Motzer, Dmitry Nosov, Tim Eisen, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a phase III randomized, open-label, multicenter trial. J Clin Oncol 2012;30:abstr 4501.