Treatment of painful bladder syndrome/interstitial cystitis with botulinum toxin A: why isn’t it effective in all patients?
Introduction
Botulinum toxin is among the most potent toxins known. Fatal at sufficient doses, the controlled use of botulinum toxin has been demonstrated to be an effective treatment for a variety of disorders, including those of the lower urinary tract (LUT). The therapeutic use of botulinum toxin A (BTA) to treat LUT disorders was first described in 1988 (1). Since that time, BTA has been used to treat a variety of LUT disorders, including painful bladder syndrome/interstitial cystitis (PBS/IC). The outcome of treatment of PBS/IC symptoms with BTA has varied, and this may relate to the heterogeneity of causes underlying PBS/IC, plasticity of the nervous system induced by PBS/IC, and disparity of effects of BTA in individual patients.
This review discusses potential mechanisms of action of BTA, locations at which BTA may have an effect, and emerging evidence that chronic PBS/IC may induce plasticity within the central nervous system (CNS) that results in persistence of symptoms. Interestingly, there has also been an increase in descriptions of transport of BTA to the CNS and its activity within the CNS. Although it is recognized that there are multiple formulations of BTA and that all are not identical in potency and biological availability (2), BTA is used as an abbreviation herein to refer to all commercially available forms of BTA.
Brief overview of mechanism
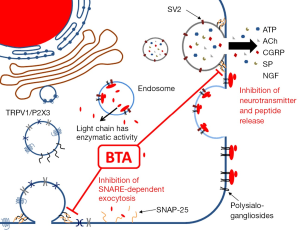
BTA is a neurotoxin produced by the bacteria Clostridium botulinum (3). Several serotypes of the toxin are produced, among which type A is the most potent (4). BTA is a zinc-dependent endopeptidase, which binds to and is taken up by, neurons via a high-affinity interaction with synaptic vesicle protein 2 (SV2) (5). Additionally, there are low-affinity interactions with polysialogangliosides on the surface of the cell that facilitate entry of BTA (6). The intracellular substrate of the type A toxin used clinically is the synaptosomal-associated protein of 25 kDa (SNAP-25), a member of the SNARE protein superfamily necessary for docking and fusion of synaptic vesicles that mediate release of neurotransmitters, including acetylcholine (ACh) (Figure 1). It is extremely potent, demonstrating a ~50 pM IC50 for inhibition of potassium-induced substance P (SP) release in primary cultures of rat dorsal root ganglia neurons (3). BTA poses a serious health risk with respect to accidental exposure (i.e., botulism), but the toxin has been utilized therapeutically, initially for treatment of movement disorders such as facial spasm, cerebral palsy, and spasticity, as well as treatment of chronic pain (4). More recently, BTA has been utilized to treat LUT dysfunctions, including incontinence (7) and detrusor overactivity (8), which have not responded to other medications. It was initially thought to act via inhibition of ACh release from efferent nerves, thereby dampening smooth muscle contractions (9). Importantly, its effects can last up to several months (4), but it does not appear to kill the neurons that absorb the toxin (10). Evidence is accumulating that BTA is also working by other mechanisms and in other cell types, but this idea has only recently been explored. Here we will briefly review the existing evidence of BTA acting on various cell types in the LUT, as well as clinical data that may help explain why it has not been effective in all patients.
Evidence of BTA effects on afferent nerves/sensation
Clinically, the sensation of urgency is thought to be directly related to increased afferent activity. BTA injections are known to reduce episodes of urgency in patients with LUT dysfunction (11), and may therefore be expected to influence afferent activity, either directly or indirectly (Figure 2). Increased afferent activity is thought to play an important role in a number of LUT pathologies, including PBS/IC, overactive bladder, and spinal cord injury (SCI), and may therefore explain the efficacy of BTA in these patients.

Two types of nerve fibers transmit sensory information from the bladder: unmyelinated C-fibers that are primarily nociceptive in humans and largely innervate the suburothelium, as well as lightly myelinated Aδ-fibers that are mostly low-threshold, mechanosensative fibers that primarily innervate the detrusor smooth muscle (12). These fibers travel primarily within the pelvic and hypogastric nerves. C-fibers are normally silent in the absence of pathology and do not play a role in the micturition reflex (13). C-fibers become active in pathological conditions, contributing to bladder overactivity (increasing afferent drive, resulting in hyperreflexia) and transmission of painful stimuli (13). One mechanism by which this happens is alterations in potassium channels resulting in increased excitability (14). This may be a result of increased expression of nerve growth factor (NGF), since chronic NGF administration in rats mimics this effect (15). In addition, increases in receptor expression of purinergic (P2X3) and transient receptor potential channels (in particular TRPV1, which responds to a number of noxious stimuli including capsaicin) in the suburothelium have been demonstrated in humans with neurogenic detrusor overactivity (NDO) relative to controls (16), which is reversed following BTA treatment (17).
C-fibers also release peptides, such as calcitonin gene-related peptide (CGRP) and SP, which promote inflammation and are upregulated in patients with PBS/IC (18-20). BTA has been shown to attenuate the enhanced release of these peptides from isolated bladders of rats with acute HCl-induced injury (21,22), and BTA decreased SP (21) and CGRP (23) expression in rats with chronic bladder inflammation induced by cyclophosphamide (CYP). BTA has also been shown to suppress potassium-induced SP release from primary cultures of rat dorsal root ganglia neurons (3), as previously mentioned.
TRPV1 is involved in pain transmission, and its expression is used to differentiate C- from Aδ-fiber afferents innervating the bladder. In bladder biopsies from PBS/IC patients, severity of inflammation was correlated with higher TRPV1-immunoreactive nerve fiber density in the suburothelium, as well as increased NGF levels (24), which were both significantly increased relative to controls. This is consistent with animal models of LUT pathology, and supports the idea that silent C-fiber activation is responsible for increased afferent activity in a number of pathologies. BTA has been shown to reduce the elevated levels of TRPV1, as well as NGF, in a rat model of bladder outlet obstruction (25). BTA injections into the detrusor caused a progressive decrease in elevated suburothelial P2X3 and TRPV1 expression (10), and these eventually returned to normal levels. It has also been shown that trafficking of TRPV1 to the plasma membrane is a SNARE-dependent process disrupted by BTA (26), suggesting that alteration of receptor expression may be another mechanism by which BTA exerts its effects.
Consistent with the observed effect of BTA on TRPV1, BTA has been shown to provide analgesia against acetic acid-induced bladder pain in rats (22). This study also showed that animals treated with BTA had less CGRP immunoreactivity than controls in response to intravesical acetic acid, again demonstrating an effect of BTA on neuropeptide release from afferent nerves in response to noxious stimuli.
Direct measurement of afferent firing with simultaneous bladder pressure recording in mice showed that BTA attenuated both low- and high-threshold afferent unit firing during distension (27), suggesting that it acts on both Aδ- and C-fibers. This study also demonstrated that acute BTA administration resulted in a 5-fold increase in luminal nitric oxide (NO) above basal levels 1 hour later. A similar study in SCI rats demonstrated a significant increase in hypoosmotic stretch-induced NO release 2 days after BTA treated vs. untreated SCI rats (28). This increase in NO may dampen afferent activity indirectly via inhibitory effects on myofibroblasts or the urothelium (29). Conversely, there was a decrease in neuronal NO synthase immunoreactivity following injection of BTA into the rat parotid gland (30). This may suggest that the increase in bladder NO content was a result of BTA effects on the urothelium. In the bladder, the balance between inhibitory NO and excitatory adenosine triphosphate (ATP) has been proposed to modulate afferent activity, and it appears that BTA helps to normalize that balance (28).
Less direct measures of afferent activity come from cystometry data, in which endpoints such as number of non-voiding contractions have been interpreted as indirect measurements of afferent activity. Following transection SCI at the T4 level, intravesical BTA given 48 hours before cystometry suppressed maximal voiding pressure and decreased the number of non-voiding contractions (31,32). BTA effectively prevents the increased NGF levels in both the spinal cord and the bladder in response to SCI (31), which is likely an important mechanism by which it suppresses afferent activity in this model. This study also demonstrated that increased arterial pressure and bradycardia in response to bladder distension, a pathological reflex termed autonomic dysreflexia, was also suppressed with BTA via an inhibition of afferent activity.
Evidence of BTA effects on urothelium
The urothelium, in addition to providing a permeability barrier to the urine, is thought to be able to sense and transmit information about fullness, pH, temperature, and infection. It contains a large number of receptors and channels for sensing the luminal environment, and releases a number of mediators in response to those changes (33). Defects in sensation or signaling by the urothelium are thought to play a role in various diseases, including PBS/IC (34).
ATP is released from the urothelium in response to stretch, which has been demonstrated in vitro in normal rabbit bladders (35), as well as primary cultures of human urothelial cells (36). The latter study also showed that urothelial cells from patients with PBS/IC released more ATP than normal controls, both at rest and in response to stretch, which was also seen in cultured urothelial cells from cats with feline interstitial cystitis (37). This urothelial-released ATP acts on purinergic receptors (primarily P2X3) on afferent nerves (38), and transmits information on bladder fullness. BTA has been shown to normalize this increased ATP release in response to stretch in SCI rats (28).
BTA has been shown to diffuse from detrusor injection sites to the suburothelium (39), where it may act on the urothelium or myofibroblasts in addition to neurons. SV2 immunoreactivity has been shown in cultured rat and human urothelial cells (40); however, it was not seen in urothelium from normal cadaveric human organ donors (41). To our knowledge, no study has examined whether SV2 is expressed in human urothelium as a result of pathology.
Intravesical administration of BTA is effective in animal preparations, in which it reduces stretch-induced ATP release into the bladder lumen (presumably from the urothelium) in both rats (40) and mice (27). In another rat study of intravesical delivery, BTA packaged in lipid micelles (liposomes) was more effective than BTA alone in minimizing acetic acid-induced changes in intercontractile interval and maximal voiding pressure (22). Taken together, these lines of evidence suggest that BTA has actions on the release of mediators from the urothelium, which are upregulated in conditions such as PBS/IC. Interestingly, ACh release from the urothelium does not appear to be vesicular (42), in contrast to release from cholinergic nerves. This may permit sensory information on fullness to be conveyed despite disruption of vesicular release of ATP by BTA.
Evidence of BTA effects on detrusor smooth muscle
In early studies, the presumed mechanism of action of BTA was thought to be primarily on efferent signals, via inhibition of vesicular ACh release from the neuromuscular junction. SV2 expression is lacking on detrusor smooth muscle (41), and exocytosis of mediators from smooth muscle cells is not known to play a role in micturition. Intravesical delivery of BTA to an ex vivo mouse bladder preparation showed no effect on bladder compliance, despite effectively dampening afferent signaling (27), suggesting that passive mechanical properties of the bladder were not acutely altered by BTA. This is consistent with studies in SCI rats showing no decreases in contraction amplitude with cystometry after intravesical delivery of BTA (43). Another study using isolated detrusor smooth muscle strips from guinea pig and mouse showed no effect of BTA incubation of up to 72 hours on contractile response to electric field stimulation (44), which may suggest that acute effects of BTA in vitro may be largely due to effects on afferent activity. A study of rat bladder strips showed only a modest effect of BTA after 2 hours of a stimulation protocol designed to promote BTA uptake; however, the mucosa was not removed from the muscle strips in this study, allowing for the possibility that the effects seen were a result of BTA on the urothelium (45). In summary, experimental evidence in animal models shows little evidence of a direct effect of BTA on detrusor smooth muscle.
Evidence of BTA effects on suburothelial myofibroblasts
Myofibroblasts (also referred to as interstitial cells, thought to be functionally similar to the interstitial cells of Cajal in the gut) are located throughout the bladder wall, but in the suburothelium are located in close proximity to axonal varicosities of both afferent and efferent nerves (46). Extensive coupling via connexin 43 gap junctions creates a functional syncytium that helps coordinate and synchronize detrusor smooth muscle function (47). Physical contact with nearby myofibroblasts enhances the response to ATP (47). They are thought to function as a variable gain amplifier, integrating information on stretch prior to relaying it to afferent nerves. NO is thought to act to attenuate afferent information relayed by myofibroblasts (29)
Dysfunctional myofibroblasts can lead to increased afferent activity. For instance, connexin 43-mediated electrical coupling is upregulated in patients with both neurogenic and idiopathic detrusor overactivity (48). However, BTA treatment did not appear to affect connexin 43 expressions in these patients (48). There is currently no evidence that myofibroblasts express SV2, or take up BTA. However, myofibroblast activity is modulated by NO and ATP, and thus indirectly influenced by BTA treatment. BTA increased NO levels while preventing ATP release, which resulted in decreased afferent firing, as demonstrated using nerve recording in mice (27). This may be mediated, at least in part, by myofibroblasts.
BTA efficacy in patients
As previously mentioned, injection of BTA for treatment of LUT dysfunction was first described in 1988 by Dykstra et al. who reported its use in 11 men with detrusor-sphincter dyssynergia subsequent to SCI (1). Since that time, BTA injection has been used to treat a variety of LUT disorders, including NDO (49,50), idiopathic detrusor overactivity, bladder outflow obstruction (49,51), pelvic pain (due to a variety of causes including muscle spasm or myofascial pain) (52,53), and LUT symptoms in men with benign prostatic hyperplasia (54). Numerous publications have described cystoscopically-guided injection of BTA into the bladder wall to manage pain and other symptoms of LUT dysfunction associated with PBS/IC in patients [reviewed in (53,55-57)]. Two articles in particular have scrutinized the use of BTA for treatment of LUT disorders, including PBS/IC (56,57). In 2011, these authors reported a lack of high-level evidence reports evaluating the efficacy of BTA for treatment of PBS/IC (56). In an updated study published in 2014 that evaluated publications on this topic since the previous study (56), the same authors identified 14 reports that provided sufficient data to assess the level of evidence, and 11 of these reports were considered to present low-level evidence, and three high-level evidence, regarding the efficacy for treatment of symptoms resulting in a diagnosis of PBS/IC (57).
Direct comparisons among studies of efficacy of treatment of symptoms of BPS/IC are complicated by the inherent heterogeneity of this patient population, as well as variations in techniques used to deliver BTA to the bladder such as the number of injection sites, total amount of BTA delivered, formulation of BTA used, and other procedures (such as bladder distention) that may or may not be performed concurrent to BTA injection (53,55). These studies have typically entailed a small number of patients, some lack appropriate control groups, and the duration of study after injection of BTA varies. Further, multiple variants of BTA are commercially available (56), and these are sufficiently different in formulation and strength that they should not all be considered pharmacologically equivalent (2). The duration of improvement varies among studies, and one study was judged to provide high-level evidence of improvement in response to repeated injection of BTA at 6 months intervals (57,58). While the general conclusion of these studies is that injection of BTA into the bladder results in improvement of symptoms of BPS/IC, particularly pain, in a majority of patients, the validity of this conclusion has been called into question because of factors previously discussed, as well as well-documented placebo effects in clinical trials of treatment of symptoms of BPS/IC.
A positive response to placebo treatments is commonly reported in studies that seek to assess the efficacy of BTA for treatment of PBS/IC symptoms. A positive response to placebo by 15-30% of PBS/IC patients who participate in randomized treatment trials (59) has been described, and more recent studies of the efficacy of systemic administration of amitriptyline or adalimumab (monoclonal antibody to tumor necrosis factor-α) to PBS/IC patients reported a 45-50% response to placebo (60-62). In at least one study, the placebo effect may have been enhanced by providing patients that received placebo treatment advice, counseling and support, education, and behavioral modification, while patients in the treatment arm (adalimumab) of the protocol also received education and behavioral modification training (62).
Experiments using rodents have demonstrated a correlation of chronic bladder inflammation with plasticity of afferent nerves, as well as alterations in the relevant segments of the spinal cord (20,63-68). Examination of bladder biopsies obtained from PBS/IC patients have also provided evidence of afferent neural plasticity (18,19), and increased concentrations of trophic factors capable of stimulating neurogenesis have been identified in the bladder wall (69) and urine (70) of PBS/IC patients. For obvious reasons, less information is available regarding alterations in the spinal cords of PBS/IC patients. However, a series of articles has recently been published describing results of non-invasive evaluation of brain structure and function in PBS/IC patients.
Evidence of CNS alterations in PBS/IC patients
It has been speculated that central processing of neural input may play a significant role in symptoms of PBS/IC (71). Alterations in brain anatomy and function have been identified on magnetic resonance imaging (MRI) images obtained from patients with chronic pain associated with fibromyalgia (72,73), back pain (74,75), chronic tension type headache (76), and various other disorders (77). Differences were observed in relative abundance of gray matter in regions of the brain associated with pain processing when MRI images of patients with chronic pelvic pain were compared to those of controls without pain (78,79). In a study of 33 PBS/IC patients without comorbidities (e.g., fibromyalgia, migraines, inflammatory bowel disease, chronic fatigue syndrome, etc.) compared to 33 age- and gender-matched normal controls, a positive correlation was observed in MRI images of PBS/IC patients between increased gray matter in areas of the brain associated with nociceptive processing (right primary somatosensory cortex, bilateral superior parietal lobules, and right somatosensory cortex) and increased pain, anxiety, and LUT symptoms that was absent in controls (80). Functional MRI (fMRI) imaging of 82 female PBS/IC patients and 85 female controls revealed altered frequency in viscerosensory, somatosensory, and motor regions of the brains of PBS/IC patients relative to that observed in controls, and increased functional connectivity was most apparent in patients with pain upon bladder filling (81). Signal attenuation by water diffusion is an important contrast mechanism for interpretation of MR images, and diffusion tensor imaging has been used to map and characterize the three-dimensional structure of the brain due to spatial diffusion of water (82,83). Significant white matter abnormalities were observed by diffusion tensor imaging of brain magnetic resonance (MR) images obtained from PBS/IC patients that were not observed in controls, and a strong correlation was observed between PBS/IC symptoms and abnormalities of white matter (84). These observations are of particular interest because changes in brain white matter structure accurately predicted the transition from acute to chronic back pain in at least one study (85).
The observation of altered brain anatomy or function in PBS/IC patients may help to explain the variable response to treatment by PBS/IC patients and also offers potentially intriguing insight into mechanisms underlying response to placebo treatment in PBS/IC and similar disorders. The concept that chronic bladder irritation results in long-term alterations in the CNS is also intriguing because of studies examining the effects of peripherally-administered BTA on higher structures. Although BTA has been used to effectively treat pain associated with a wide variety of disorders, the mechanism(s) by which BTA exerts anti-nociceptic effects remains unknown (86). The most commonly accepted theory is that BTA acts at peripheral sites to suppress transmission of nociceptive stimuli (53,87-92). However, there are increasing reports that peripherally-administered BTA may be transported to the CNS and that it may be this mechanism that allows BTA to suppress pain (93-100).
Evidence of BTA effects in CNS
Radiolabeled BTA was injected into the bladders of rats, and radioactivity was evaluated in the bladder, L6-S1 dorsal root ganglia, and the L6-S1 spinal cord segments at 1, 3, and 6 hours after injection of BTA (93). Concentrations of radiolabeled BTA increased over time in both the L6-S1 dorsal root ganglia and L6-S1 spinal cord segments (93). Intrathecal administration of BTA effectively suppressed pain in rats arising from CYP-induced bladder inflammation (23) or abdominal pain in rats induced by intraperitoneal injection of 1% acetic acid or colitis resulting from intraluminal infusion of capsaicin (101). BTA has been shown to suppress release of neurotransmitters by inhibition of SNARE complex proteins, and cleavage of synaptosomal-associated protein of 25 kDa (SNAP-25; a component of the SNARE complex) has been used as an indicator of BTA activity (102). Intrathecal injection of BTA at the L5-6 region of the spinal cord in rats with cystitis resulted in increased cleavage of SNAP-25 in the dorsal horn of the L6 segment of the spinal cord associated with neurons penetrating through lamina X, as well as evidence of cleavage of SNAP-25 in the L6 ventral horn around the cell bodies of motor neurons (23). It is interesting to note that a similar extent of cleavage of SNAP-25 within the L6 spinal cord segment persisted was observed 30 days after intrathecal BTA, and that injection of BTA into the cysterna magna had no effect on CYP-induced visceral pain (23). These findings strongly suggest that the anti-nociceptive effect of BTA in this model was the result of activity within the spinal cord and that the CNS effects of BTA may be prolonged.
Further evidence of central action of peripherally-administered BTA is provided by reports of bilateral analgesia subsequent to unilateral BTA administration to animals in which pain was induced by bilateral intramuscular injection of acidic saline (103). Neuronal transport of BTA by afferent fibers to the CNS is supported by the observation that treatment of afferent nerves or ganglia with the axonal transport inhibitor colchicine blocked the analgesic effects of BTA (97,104). Interestingly, injection of colchicine into the ipsilateral sciatic nerve proximal to the site of injury inhibited bilateral anti-nociceptive effects of unilateral injection of BTA, but injection of colchicine into the contralateral sciatic nerve relative to the site of BTA treatment failed to prevent BTA-induced analgesia, indicating that BTA does not have to reach peripheral nerve endings to exert an anti-nociceptive effect (103).
There appears to be less experimental data regarding neuronal transport of BTA to the brain, but when BTA is injected directly into the eye or brain, it is distributed by neuronal transport and exerts effects similar to those reported in peripheral tissue as evidenced by cleavage of SNAP-25 (98,99). It appears highly probable that BTA is transported to the brain. In 2010, the FDA approved BTA for prophylactic treatment of chronic migraine headaches. The studies cited in support of this approval reported the results of treatment of patients with a total dose of 155 units distributed among 31 injection sites (105,106). A meta-analysis of studies describing the efficacy of BTA for treatment of various categories of chronic head ache confirmed that BTA is an effective treatment alternative in many patients and also provides further support for the concept that BTA may exert its analgesic effects via action within the CNS (107).
Summary
BTA clearly has the potential to manage symptoms of PBS/IC; however, results vary among patients. This appears to reflect a continued lack of understanding of the causes of initiation and persistence of PBS/IC. As with other chronic disorders, it is highly probable that there is a progressive change in mechanisms underlying symptoms of PBS/IC that is accompanied by anatomical and physiological changes that occur as PBS/IC persists. The observation of changes in anatomy and function of the gray and white matter in the brains of PBS/IC patients suggests that more comprehensive assessment of patients is required to identify treatment options that are tailored to the needs of individual patients. These observations may also underlie the relatively strong response to placebo treatment of PBS/IC symptoms that has been reported.
BTA appears to have significant potential for effective treatment of PBS/IC, but it remains unlikely that a single therapeutic approach will provide relief in all patients. Multiple mechanisms of action with a number of cell types may account for some of the interindividual variability. Recent findings of CNS changes in PBS/IC patients strongly support the concept that a more sophisticated and comprehensive understanding of mechanisms resulting in symptoms of PBS/IC, as well as other disorders characterized by chronic pain, are crucial to identifying improved therapeutic options.
Acknowledgements
Funding: NS Lamarre is supported by T32ES007015 from the National Institute of Environmental Health Sciences.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139:919-22. [PubMed]
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003;44:165-74. [PubMed]
- Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000;38:245-58. [PubMed]
- Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol 2001;8 Suppl 5:21-9. [PubMed]
- Baldwin MR, Barbieri JT. Association of botulinum neurotoxins with synaptic vesicle protein complexes. Toxicon 2009;54:570-4. [PubMed]
- Cruz F. Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn 2014;33:31-8. [PubMed]
- Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174:196-200. [PubMed]
- Kuo HC. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004;63:868-72. [PubMed]
- Jankovic J, Brin MF. Therapeutic uses of botulinum toxin. N Engl J Med 1991;324:1186-94. [PubMed]
- Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49:644-50. [PubMed]
- Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs. onabotulinumtoxina for urgency urinary incontinence. N Engl J Med 2012;367:1803-13. [PubMed]
- Takeda M, Mochizuki T, Yoshiyama M, et al. Sensor mechanism and afferent signal transduction of the urinary bladder: special focus on transient receptor potential ion channels. LUTS 2010;2:51-60.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 2008;9:453-66. [PubMed]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 1999;19:4644-53. [PubMed]
- Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 2006;26:10847-55. [PubMed]
- Brady CM, Apostolidis A, Yiangou Y, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol 2004;46:247-53. [PubMed]
- Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 2005;174:977-82; discussion 982-3. [PubMed]
- Marchand JE, Sant GR, Kream RM. Increased expression of substance P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br J Urol 1998;81:224-8. [PubMed]
- Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol 1995;75:744-50. [PubMed]
- Yu SJ, Xia CM, Kay JC, et al. Activation of extracellular signal-regulated protein kinase 5 is essential for cystitis- and nerve growth factor-induced calcitonin gene-related peptide expression in sensory neurons. Mol Pain 2012;8:48. [PubMed]
- Lucioni A, Bales GT, Lotan TL, et al. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int 2008;101:366-70. [PubMed]
- Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 2004;172:1529-32. [PubMed]
- Coelho A, Oliveira R, Rossetto O, et al. Intrathecal administration of botulinum toxin type A improves urinary bladder function and reduces pain in rats with cystitis. Eur J Pain 2014;18:1480-9. [PubMed]
- Liu BL, Yang F, Zhan HL, et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int 2014;92:202-8. [PubMed]
- Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 2011;78:721.e1-721.e6.
- Morenilla-Palao C, Planells-Cases R, García-Sanz N, et al. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem 2004;279:25665-72. [PubMed]
- Collins VM, Daly DM, Liaskos M, et al. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int 2013;112:1018-26. [PubMed]
- Smith CP, Gangitano DA, Munoz A, et al. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int 2008;52:1068-75. [PubMed]
- Smet PJ, Jonavicius J, Marshall VR, et al. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 1996;71:337-48. [PubMed]
- Ellies M, Schütz S, Quondamatteo F, et al. Immunohistochemical investigations of the influence of botulinum toxin A on the immunoreactivity of nNOS in the parotid gland of the rat. J Oral Maxillofac Surg 2006;64:397-401. [PubMed]
- Elkelini MS, Bagli DJ, Fehlings M, et al. Effects of intravesical onabotulinumtoxinA on bladder dysfunction and autonomic dysreflexia after spinal cord injury: role of nerve growth factor. BJU Int 2012;109:402-7. [PubMed]
- Behr-Roussel D, Oger S, Pignol B, et al. Minimal effective dose of dysport and botox in a rat model of neurogenic detrusor overactivity. Eur Urol 2012;61:1054-61. [PubMed]
- Birder LA. Urothelial signaling. Auton Neurosci 2010;153:33-40. [PubMed]
- Birder LA. Urinary bladder, cystitis and nerve/urothelial interactions. Auton Neurosci 2014;182:89-94. [PubMed]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol 1997;505:503-11. [PubMed]
- Sun Y, Keay S, De Deyne PG, et al. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 2001;166:1951-6. [PubMed]
- Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 2003;285:F423-9. [PubMed]
- Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000;407:1011-5. [PubMed]
- Schulte-Baukloh H, Knispel HH. A minimally invasive technique for outpatient local anaesthetic administration of intradetrusor botulinum toxin in intractable detrusor overactivity. BJU Int 2005;95:454. [PubMed]
- Hanna-Mitchell AT, Wolf-Johnston AS, Barrick SR, et al. Effect of botulinum toxin A on urothelial-release of ATP and expression of SNARE targets within the urothelium. Neurourol Urodyn 2015;34:79-84. [PubMed]
- Coelho A, Dinis P, Pinto R, et al. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol 2010;57:884-90. [PubMed]
- Lips KS, Wunsch J, Zarghooni S, et al. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 2007;51:1042-53. [PubMed]
- Munoz A, Somogyi GT, Boone TB, et al. Central inhibitory effect of intravesically applied botulinum toxin A in chronic spinal cord injury. Neurourol Urodyn 2011;30:1376-81. [PubMed]
- Howles S, Curry J, McKay I, et al. Lack of effectiveness of botulinum neurotoxin A on isolated detrusor strips and whole bladders from mice and guinea-pigs in vitro. BJU Int 2009;104:1524-9; discussion 1529-30. [PubMed]
- Smith CP, Boone TB, de Groat WC, et al. Effect of stimulation intensity and botulinum toxin isoform on rat bladder strip contractions. Brain Res Bull 2003;61:165-71. [PubMed]
- Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 2003;91:89-93. [PubMed]
- Fry CH, Sui GP, Kanai AJ, et al. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn 2007;26:914-9. [PubMed]
- Roosen A, Datta SN, Chowdhury RA, et al. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur Urol 2009;55:1440-8. [PubMed]
- Truzzi JC. Overactive Bladder Syndrome, Detrusor Overactivity and the Botulinum Toxin. Rev Recent Clin Trials 2015;10:128-34. [PubMed]
- Karsenty G, Denys P, Amarenco G, et al. Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic literature review. Eur Urol 2008;53:275-87. [PubMed]
- Malde S, Dowson C, Fraser O, et al. Patient experience and satisfaction with Onabotulinumtoxin A for refractory overactive bladder. BJU Int 2015;116:443-9. [PubMed]
- Adelowo A, Hacker MR, Shapiro A, et al. Botulinum toxin type A (BOTOX) for refractory myofascial pelvic pain. Female Pelvic Med Reconstr Surg 2013;19:288-92. [PubMed]
- Jhang JF, Kuo HC. Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection. Toxins (Basel) 2015;7:2232-50. [PubMed]
- Sacco E, Bientinesi R, Marangi F, et al. Patient-reported outcomes in men with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) treated with intraprostatic OnabotulinumtoxinA: 3-month results of a prospective single-armed cohort study. BJU Int 2012;110:E837-44. [PubMed]
- Jhang JF, Jiang YH, Kuo HC. Potential therapeutic effect of intravesical botulinum toxin type A on bladder pain syndrome/interstitial cystitis. Int J Urol 2014;21 Suppl 1:49-55. [PubMed]
- Mangera A, Andersson KE, Apostolidis A, et al. Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol 2011;60:784-95. [PubMed]
- Mangera A, Apostolidis A, Andersson KE, et al. An updated systematic review and statistical comparison of standardised mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur Urol 2014;65:981-90. [PubMed]
- Kuo HC. Repeated onabotulinumtoxin-a injections provide better results than single injection in treatment of painful bladder syndrome. Pain Physician 2013;16:E15-23. [PubMed]
- Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol 2003;170:810-5. [PubMed]
- Foster HE Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol 2010;183:1853-8. [PubMed]
- Bosch PC. A randomized, double-blind, placebo controlled trial of adalimumab for interstitial cystitis/bladder pain syndrome. J Urol 2014;191:77-82. [PubMed]
- Bosch PC. Examination of the significant placebo effect in the treatment of interstitial cystitis/bladder pain syndrome. Urology 2014;84:321-6. [PubMed]
- Frias B, Allen S, Dawbarn D, et al. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience 2013;234:88-102. [PubMed]
- Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 2008;507:1379-92. [PubMed]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 2000;161:273-84. [PubMed]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 2000;278:R1027-39. [PubMed]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 2001;21:125-38. [PubMed]
- Merrill L, Girard BM, May V, et al. Transcriptional and translational plasticity in rodent urinary bladder TRP channels with urinary bladder inflammation, bladder dysfunction, or postnatal maturation. J Mol Neurosci 2012;48:744-56. [PubMed]
- Lowe EM, Anand P, Terenghi G, et al. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 1997;79:572-7. [PubMed]
- Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 1999;161:438-41; discussion 441-2. [PubMed]
- Clemens JQ. Male and female pelvic pain disorders--is it all in their heads? J Urol 2008;179:813-4. [PubMed]
- Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002;46:1333-43. [PubMed]
- Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain 2007;132 Suppl 1:S109-16. [PubMed]
- Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5. [PubMed]
- Schmidt-Wilcke T, Leinisch E, Gänssbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 2006;125:89-97. [PubMed]
- Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology 2005;65:1483-6. [PubMed]
- Baliki MN, Mansour AR, Baria AT, et al. Functional reorganization of the default mode network across chronic pain conditions. PLoS One 2014;9:e106133. [PubMed]
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 2012;153:1006-14. [PubMed]
- Farmer MA, Chanda ML, Parks EL, et al. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol 2011;186:117-24. [PubMed]
- Kairys AE, Schmidt-Wilcke T, Puiu T, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol 2015;193:131-7. [PubMed]
- Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 2014;192:947-55. [PubMed]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247-54. [PubMed]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259-67. [PubMed]
- Farmer MA, Huang L, Martucci K, et al. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. J Urol 2015;194:118-26. [PubMed]
- Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain 2013;154:2160-8. [PubMed]
- Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol 2014;119-120:39-59. [PubMed]
- Kuo HC, Jiang YH, Tsai YC, et al. Intravesical botulinum toxin-A injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment - A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourol Urodyn 2015. [Epub ahead of print]. [PubMed]
- Smith CP, Radziszewski P, Borkowski A, et al. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology 2004;64:871-5; discussion 875. [PubMed]
- Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord 2011;17 Suppl 1:S28-33. [PubMed]
- Francisco GE, Tan H, Green M. Do botulinum toxins have a role in the management of neuropathic pain?: a focused review. Am J Phys Med Rehabil 2012;91:899-909. [PubMed]
- Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology 2013;306:124-46. [PubMed]
- Wang T, Martin S, Papadopulos A, et al. Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type a. J Neurosci 2015;35:6179-94. [PubMed]
- Papagiannopoulou D, Vardouli L, Dimitriadis F, et al. Retrograde transport of radiolabelled botulinum neurotoxin type A to the CNS after intradetrusor injection in rats. BJU Int 2015. [Epub ahead of print]. [PubMed]
- Bach-Rojecky L, Dominis M, Lacković Z. Lack of anti-inflammatory effect of botulinum toxin type A in experimental models of inflammation. Fundam Clin Pharmacol 2008;22:503-9. [PubMed]
- Drinovac V, Bach-Rojecky L, Matak I, et al. Involvement of µ-opioid receptors in antinociceptive action of botulinum toxin type A. Neuropharmacology 2013;70:331-7. [PubMed]
- Marinelli S, Vacca V, Ricordy R, et al. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One 2012;7:e47977. [PubMed]
- Filipović B, Matak I, Bach-Rojecky L, et al. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS One 2012;7:e29803. [PubMed]
- Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci 2008;28:3689-96. [PubMed]
- Restani L, Antonucci F, Gianfranceschi L, et al. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J Neurosci 2011;31:15650-9. [PubMed]
- Restani L, Giribaldi F, Manich M, et al. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog 2012;8:e1003087. [PubMed]
- Drinovac V, Bach-Rojecky L, Babić A, et al. Antinociceptive effect of botulinum toxin type A on experimental abdominal pain. Eur J Pharmacol 2014;745:190-5. [PubMed]
- Chen S, Kim JJ, Barbieri JT. Mechanism of substrate recognition by botulinum neurotoxin serotype A. J Biol Chem 2007;282:9621-7. [PubMed]
- Bach-Rojecky L, Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav 2009;94:234-8. [PubMed]
- Matak I, Bach-Rojecky L, Filipović B, et al. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011;186:201-7. [PubMed]
- Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010;30:793-803. [PubMed]
- Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804-14. [PubMed]
- Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA 2012;307:1736-45. [PubMed]


