Role of hormonal therapy for prostate cancer: perspective from Japanese experiences
Introduction
Hormonal therapy for the treatment of prostate cancer was first introduced about 70 years ago by Huggins and Hodges (1). At that time, methods of hormonal therapy consisted of surgical castration and/or estrogen therapy. Such treatment was only used in cases of advanced prostate cancer, because surgical castration results in permanent androgen deprivation. However, the development of luteinizing hormone releasing hormone (LH-RH) analog allowed us to compensate for androgen deprivation in such cases, and the indications for hormonal therapy have therefore changed. Hormonal therapy is frequently used as neoadjuvant and/or adjuvant therapy in patients undergoing radical prostatectomy or radiotherapy. Furthermore, hormonal therapy is sometimes used as the primary treatment for localized prostate cancer, especially in aged patients. Thus, hormonal therapy has been widely used. However, it has recently been the subject of criticism that it shows minimal effectiveness (2), it may reduce patients’ quality of life (QOL), and induce adverse effects (3,4). On the other hand, next-generation hormonal drugs have provided new strategies for hormonal therapy to overcome advanced prostate cancer.
This article presents a review of the possible roles of hormonal therapy for prostate cancer based upon experience in Japan.
Theoretical background of hormonal therapy
Most prostate cancer cells express androgen receptor (AR). In prostate cancer cells, dihydrotestosterone (DHT) is converted from testosterone produced in the testis. DHT, which binds with androgen receptor (AR) in the nuclei of prostate cancer cells, activates androgen-responsive genes, and finally plays a major role in the proliferation of prostate cancer cells. AR is a member of the steroid hormone receptor superfamily, and is activated by androgens resulting in androgenic effects on androgen-target organs. Therefore, androgen deprivation by surgical or medical castration could theoretically suppress growth of most prostate cancer cells, because serum testosterone concentrations fall to less than 50 ng/mg after castration. However, testosterone and DHT are also converted from dehydroepiandrosterone (DHEA) and androstenedione secreted from the adrenal gland, and it has been reported that approximately 40% of androgen in prostate tissue is derived from the adrenal gland (5) (intracrine hormone synthesis). We also showed that approximately 25% of testosterone in prostate cancer tissue remained after castration (6). These results suggested that ADT for prostate cancer requires not only surgical or medical castration using LH-RH analog but also antiandrogen agents. Based upon these findings, combined androgen blockade (CAB) using castration and antiandrogen agents was advocated. On the other hand, antiandrogen agents block the activities of androgens by various mechanisms, i.e., suppression of LH secretion in the pituitary gland, inhibition of androgen binding with AR, and suppression of androgen-AR complex translocation to the nucleus. Therefore, it is possible that the different clinical outcomes of CAB treatment are due to the various types of antiandrogen agent used (7).
Role of hormonal therapy in treatment of advanced prostate cancer
Hormonal therapy is still the first choice for treatment of advanced prostate cancer, because it is useful in more than 90% of cases of advanced prostate cancer. There has been some controversy whether CAB is superior to castration alone. Recently, the results of a phase 3 randomized controlled trial of CAB in advanced prostate cancer showed that LH-RH analog +80 mg of bicalutamide was more effective than LH-RH analog alone, with favorable safety profiles and cost-effectiveness and without deterioration of QOL (8). Although the effectiveness of CAB treatment has been confirmed, most patients with advanced prostate cancer unfortunately experience relapse, a condition known as hormone refractory prostate cancer (HRPC). Chemotherapy using docetaxel is the standard treatment in such cases of relapsed prostate cancer after primary hormonal therapy failure. Other modalities of hormonal therapy using other antiandrogen agents (9), glucocorticoids, estrogens, or ketoconazole can be used as the second or third line of hormonal therapy, and have frequently been effective in so-called HRPC. Therefore, HRPC was shown to not be necessarily hormone-independent, and therefore it has been renamed castration-resistant prostate cancer (CRPC).
Mechanisms of relapse after first line hormonal therapy
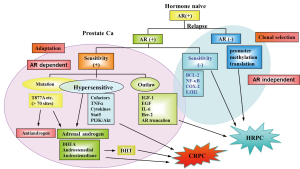
Relapsed prostate cancers can be divided into 3 types after first line hormonal therapy, as shown in Figure 1. The first is AR signal-independent cancer, which can survive without the AR signal. This is the real HRPC, which is indicated for chemotherapy. The second is AR signal-dependent but ligand-independent. The third group still has the ligand-dependent AR signal. This type is CRPC, because it shows no more response to conventional hormonal therapy using CAB. The mechanisms of CRPC are thought to be as follows. First, these lesions are thought to have greater sensitivity of AR to androgen. AR signaling can be amplified by AR overexpression, AR mutations, or changes in AR-interacting factors, such as cofactors. With such increased sensitivity of AR, even low levels of androgen can induce AR activation. The second mechanism of CRPC is intraprostatic formation of androgens. As mentioned above (5,6), approximately 25-40% of DHT remain in castrated prostate tissue in which enzymes that convert progesterone to androgen were shown to be overexpressed. This DHT is converted from precursor steroids, which are derived from the adrenal gland and peripheral tissues. This relatively low concentration of DHT may be sufficient to stimulate AR signaling via increased sensitivity of AR.
Treatment of CRPC
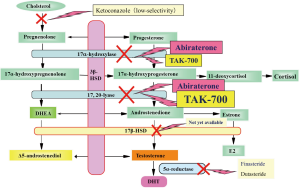
Given the several mechanisms of CRPC (10), many therapeutic agents have been developed. The first target in CRPC is inhibition of androgen biosynthesis in prostatic cancer tissues (Figure 2).
As ketoconazole completely inhibits androgen synthesis, it could artificial adrenal deficiency. However, it could be useful when used carefully supplemented with prednisone (11). As estrogen is known to induce adverse cardiovascular effects, its use has been limited. However, it has been reported to be very effective in Japanese CRPC patients (12). As flutamide sometimes induces hepatic dysfunction, its use as first line treatment has been decreasing. However, flutamide not only has antiandrogenic effects but also suppressed androgen biosynthesis (7). Therefore, with close attention, the above-mentioned drugs should be reconsidered for use as second line hormonal therapy for CRPC, at least until the next-generation hormonal drugs described below become available.
Inhibition of CYP17 is promising, because upregulation of CYP17 expression has been demonstrated in CRPC tissues (13). CYP17 catalyzes two essential reactions in androgen biosynthesis, 17-hydroxylase and C17,20 lyase (14-16). Three novel selective inhibitors of CYP17 are currently under development. Abiraterone acetate is a small-molecule CYP17A1 inhibitor. As abiraterone acetate inhibits both 17-hydroxylase and C17,20 lyase, glucocorticoid replacement is necessary. Recently, clinical trials to compare the effectiveness of abiraterone plus prednisone with those of prednisone plus placebo in CRPC patients previously treated with docetaxel showed significant improvement in overall survival of patients treated with abiraterone plus prednisone (17,18) and the US FDA has approved its use in treatment of advanced prostate cancer progressing after docetaxel treatment. TAK-700 (orteronel) is a more selective inhibitor of CYP17, because inhibition of C17,20 lyase is more potent than that of 17-hydroxylase (19). Thus, glucocorticoid replacement may be unnecessary or its requirement may be only minimal in comparison to patients treated with abiraterone. Phase 3 studies of TAK-700 are currently underway in both CRPC patients who have received chemotherapy and CRPC patients who are chemotherapy naive. TOK-001 is also a selective inhibitor of CYP17 (20). This compound also downregulates AR expression.
MDV3100 is a novel second generation antiandrogen. MDV3100 has greater binding affinity for AR to inhibit DNA binding of androgens to AR (21). MDV3100 also inhibits nuclear translocation of androgens. Furthermore, it can inhibit the association of AR and DNA within the cell nucleus. In a phase 1/2 multicenter study of 140 patients with CRPC, MDV3100 showed overall ≥50% PSA decrease in 56% of patients (22). The AFFIRM phase 3 study was conducted in men with CRPC who had progressed after treatment with docetaxel-based chemotherapy. The trial stopped in November 2011 because a planned interim analysis showed a 37% reduction in the risk of death with MDV3100 over placebo (median, 18.4 vs. 13.6 months; HR, 0.631).
Treatment for AR signal-dependent but ligand-independent CRPC
Although intraprostatic androgen concentration can be reduced by next-generation hormonal drugs, AR can sometimes autonomously maintain transcription ability without ligand. Molecular targeted therapy may be indicated in such cases in which AR-interacting proteins upregulate transcriptional activity of AR by cross-talk.
On the other hand, an AR splice variant has autonomous transcription ability (23). However, variant AR is thought to bind to AR-binding domain as a heterodimer with intact AR. Therefore, MDV3100, which inhibits ligand binding to AR, may be effective for such variant AR-induced CRPC (24).
Ideally, direct AR-targeted therapy may be most effective; such a drug has recently been reported (25), and the results of clinical trials are awaited.
How should we select the second line treatment after relapse of the first line hormonal therapy?
Many next-generation hormonal drugs are now available. On the other hand, new chemotherapeutic and immunotherapeutic drugs have also been developed. Therefore, it is very important to establish strategies for the sequential use of such drugs after relapse of the first line hormonal therapy. Many factors, including the duration of effectiveness of the first line hormonal therapy, immunohistochemical findings of re-biopsied prostate tissue, and serum & intraprostatic concentrations of androgens, may be helpful to decide on the next drug(s) to be administered. Of course, clinical trials for such purposes are required.
Role of hormonal therapy for high-risk or locally advanced localized prostate cancer
Patients with high-risk or locally advanced prostate cancer with high Gleason score, elevated PSA level, and advanced clinical stage have a high probability of treatment failure after initial management by single-treatment modalities, such as hormonal therapy (26), radical prostatectomy, external beam radiation therapy (EBRT), or brachytherapy (27,28). Therefore, it is important to establish the most effective treatment strategy for patients with high-risk prostate cancer. As high-risk patients may have locally advanced disease with direct extension and/or micrometastases, various combinations of treatments have been developed to augment cancer-specific survival. Neoadjuvant and/or adjuvant hormonal therapy offer synergistic enhancement of radiation therapy or radical prostatectomy due to induction of apoptosis. Moreover, hormonal therapy may play a role in elimination of occult systemic disease (29,30). Whereas many studies have demonstrated benefits of hormonal therapy used in conjunction with EBRT to treat locally advanced prostate cancer (31-35), questions remain, including the details of the duration, timing, and contents of hormonal therapy. The results of the Radiation Oncology Group trial (RTOG)-9202 regarding the effectiveness and adverse effects of hormone therapy are very informative (36). These results suggest that cause-specific benefits of hormone therapy may have been offset by deaths from other causes induced by hormone therapy. As the prolonged use of hormonal therapy results in increased incidence rates of adverse events, investigation of the optimal duration of hormonal therapy with maximization of clinical outcome and minimization of toxicity is a logical step in the management of localized high-risk prostate cancer. Further, we should determine which patients with high risk prostate cancer will actually benefit from hormonal therapy even if there is some compromise in QOL associated with the adverse event profile of this treatment method. Clinical trials have demonstrated the superiority of longer periods of adjuvant hormonal therapy (34). Therefore, with sufficient care to prevent adverse effects due to hormonal therapy, better outcomes may be achieved with longer periods of neoadjuvant and/or adjuvant hormonal therapy. Tri-modality treatment (EBRT + brachytherapy + hormonal therapy) has attracted attention as another method to produce better outcomes in cases of high-risk prostate cancer (37). According to the American Brachytherapy Society (ABS), brachytherapy alone is not recommended for high-risk prostate cancer but can be used as a boost in conjunction with EBRT (38). In this multimodal approach, a combination of brachytherapy and EBRT theoretically delivers a possible escalated dose to the prostate and at the same time to extracapsular cancer extension. Although the ABS provides no clear indications for neoadjuvant and/or adjuvant hormonal therapy with combination of brachytherapy and EBRT in high-risk prostate cancer, the duration of hormonal therapy could be reduced with such multi-modality radiotherapy. A new trial has just begun to investigate the optimal duration of hormonal therapy in combination with brachytherapy and EBRT (39).
In contrast to the many efforts to develop better treatment regimens for radiotherapy with hormonal therapy, there have been few clinical trials investigating the effectiveness of neoadjuvant and/or adjuvant hormonal therapy with radical prostatectomy (40). One reason for this is that early studies of neoadjuvant hormonal therapy did not confirm the improvement of overall survival despite improvements in the pathological findings. In addition, surgeons may have less interest in medical treatments, such as hormonal therapy. However, surgeons should consider the best methods for improving the results in cases of high-risk prostate cancer, because recent reports have indicated the superiority of radiotherapy for high-risk prostate cancer compared with radical prostatectomy (41). Recently Dorff et al. reported that 2 years of adjuvant androgen deprivation therapy (ADT) after radical prostatectomy resulted in an extremely low rate of disease recurrence and prostate cancer-specific death for high-risk patients in the SWOG S9921 Study (42).
Finally, it should be stressed that it may be possible to eradicate prostate cancer death even in the high-risk or locally advanced prostate cancer with appropriate use of hormonal therapy in combination with radiotherapy or radical prostatectomy. Therefore, further well-designed clinical trials are required.
Efficacy of primary hormonal therapy for localized or low-risk prostate cancer
Hormonal therapy is not recommended as the primary treatment for localized prostate cancer according to representative guidelines, such as the National Comprehensive Cancer Network (NCCN) guidelines. However, according to the Japanese cancer registration statistics, many patients with localized prostate cancer have actually been treated with primary hormonal therapy (43). Despite urologist’s explanation regarding the various treatments for localized prostate cancer, many patients select primary hormonal therapy in Japan (44). It is likely that many patients with localized prostate cancer select primary hormonal therapy because such medical treatment is more acceptable than more invasive treatments, such as surgery, at least for many Japanese patients. In addition, urologists themselves may also influence patients’ decisions because they have experience regarding the effectiveness of primary hormonal therapy.
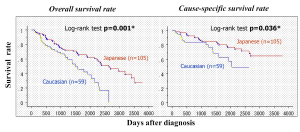
The ethnic background of patients may play an important role in the effectiveness of hormonal therapy and in susceptibility to adverse effects. The efficacy of hormonal therapy has been compared between Japanese-Americans and Caucasians living in Hawaii (45). Both groups had similar backgrounds, but both overall and cause-specific survival rates of Japanese-Americans were better than those of Caucasian subjects (Figure 3). The overall survival rate was also compared among Caucasian, Chinese, and Filipino patients living in Hawaii. The Chinese subjects showed similar trends to Japanese patients. Therefore, sensitivity of prostate cancer to hormonal therapy and susceptibility to adverse effects may differ among ethnic groups.
Akaza et al. reported that overall survival of patients with localized or locally advanced prostate cancer treated with primary hormonal therapy was equivalent to life expectancy of age-matched subjects in the healthy population (46). Before Akaza’s report Egawa et al. had already reported that primary hormonal therapy was as effective as radical prostatectomy with regard to disease-specific survival rate in localized prostate cancer (47). In their report, disease-specific survival rate at 10 years of 56 patients with well-differentiated prostate cancer treated with primary hormonal therapy was 100%. Why is the outcome of primary hormonal therapy so excellent, especially in well-differentiated prostate cancer? Kitagawa et al. analyzed the histological effects of hormonal therapy in specimens from patients treated with radical prostatectomy after neoadjuvant hormonal therapy (48). They reported that histologically cured or nearly cured patients accounted for more than 40% of the total number. In addition, the recurrence-free survival rate of patients with histologically complete apoptosis was 100%. These results suggest that some cases of localized prostate cancer could be cured by primary hormonal therapy alone. Schulman et al. also performed neoadjuvant hormonal treatment for 3 months before radical prostatectomy in patients with localized prostate cancer, and reported good histological effects (49). Labrie also reported that long-term control of about 80% of Stage B prostate cancers could be achieved with primary hormonal therapy (50).
These reports raise questions about which groups of patients would be good candidates for primary hormonal therapy. We performed a retrospective review of the efficacy of primary hormonal therapy in 628 patients with localized or locally advanced prostate cancer treated with primary hormonal therapy at 7 institutions in Japan, and attempted to predict patients in whom the disease could be controlled for long periods by primary hormonal therapy (51). Disease-specific and overall survival rates at 8 years in all patients were 89.1% and 75.0%, respectively. In addition, disease-specific survival rate at 8 years of patients given combined androgen blockade (CAB) treatment was 95.3%, which was significantly higher than that of patients treated with castration alone. We classified the patients into three risk groups based on pretreatment PSA level and Gleason score according to a modification of the D’Amico risk grouping (52). Disease-specific survival rates at 8 years of low-, intermediate-, and high-risk groups were 97.6%, 95.4%, and 78.3%, respectively. Next, we divided low- and intermediate-risk patients into two groups with PSA level <0.2 ng/mL after hormonal therapy. The time to PSA level <0.2 was within 6 months in 192 patients (good response group, Group G). These patients accounted for 30.6% of the total patient population. We classified the 139 patients in whom the PSA level did not fall below 0.2 within 6 months as the poor response group (Group P). The disease-specific survival rates at 8 years of Groups G and P were 98.9% and 94.0%, respectively. Notably, there were no cancer-related deaths during the observation period among the 133 patients in Group G receiving CAB treatment in this study. Although a randomized controlled trial may be necessary for utilization of primary hormonal therapy in patients in whom such treatment is considered more effective, based on the results of our study T1c-T3 patients with PSA level ≤20 ng/mL and Gleason score ≤7 may be good candidates for the initial hormonal therapy. These patients accounted for 52.7% of the total number of T1c-T3 patients in our study. Hormonal therapy may be suitable as the initial treatment in such patients, but changing to another curative regimen or combination therapy with radiotherapy or radical prostatectomy should be considered if the PSA value does not decrease to <0.2 ng/mL after 6 months of hormonal therapy. However, in patients in whom the PSA value drops to <0.2 ng/mL within 6 months of the commencement of hormonal therapy, continuation of the same regimen may be reasonable with careful observation.
Another preference for early-stage prostate cancer patients involves active surveillance. No study in PSA screened low-risk cancer has ever shown that treatment is better than no treatment. Therefore, further investigations are necessary to compare the disease-specific or progression-free survival rates of a low-risk group, such as Group G, with those of an active surveillance group. The PIVOT trial has recently shown that radical prostatectomy did not reduce mortality to a greater extent than observation in men with low PSA or low-risk prostate cancer. However, even cancer cells for which observation alone without treatment was at first thought to be sufficient are not always inactive after long periods. These cancer cells may become impossible to control due to malignant transformation by gene mutation during follow-up (Figure 4) (53). In addition, most patients are anxious about the status of their disease, and few are willing to rely solely on active surveillance (54). Another possible problem is the period over which hormonal therapy should be continued. Labrie et al. performed long-term hormonal therapy in stage B and C patients and discontinued the treatment in patients who did not show PSA recurrence. An increase in PSA occurred in only 2 of 33 patients with stage B and C prostate cancer who stopped treatment after continuous CAB for more than 6.5 years. In addition, seven of eight patients with localized prostate cancer who received CAB treatment continuously for 6.5-9.0 years before stopping treatment showed no PSA failure at least 5 years after cessation of CAB. CAB treatment was restarted in patients showing PSA recurrence after cessation of the initial hormonal treatment, and control was achieved again in most cases. Thus, it was concluded that CAB treatment for 7 years may be suitable in such cases. Recently, Tanaka et al. also investigated when hormonal therapy could be discontinued based on nadir PSA levels after commencement of treatment. They concluded that a relatively short period, e.g., 3 years, may be sufficient in cases in which the nadir PSA dropped to <0.01 ng/mL (55). Although intermittent hormonal therapy was reported to be useful for the treatment of advanced prostate cancer to maintain sensitivity to androgens (56), care is required in application of this treatment for localized prostate cancer as cancer that could be controlled over the long-term or may be cured by appropriate hormonal therapy (50) may progress to develop more malignant potential by incomplete androgen ablation.

According to the modified D’Amico classification reported previously (51), disease-specific and progression-free survival rates of the high-risk group treated with primary hormonal therapy at 5 years were 87.8% and 58.8%, respectively. From these results long-term control by primary hormonal therapy seems difficult in the high-risk group. However, Mizokami et al. (57) reanalyzed the previous data and showed that the results for the high-risk group are not necessarily pessimistic in patients in whom the PSA value drops below 0.2 ng/mL. They proposed that high-risk prostate cancer patients should be first treated with neoadjuvant CAB. Then, once a PSA value of <0.2 has been reached, patients with favorable parameters (Gleason score ≤6, pretreatment PSA ≤20, time to PSA <0.2 ng/mL within 6 months after commencement of hormonal therapy) are likely to have reduced likelihood (<25%) of relapse at 10 years after commencement of CAB. Therefore, such patients could select any treatment option, e.g., surgery, radiotherapy, or primary hormonal therapy. However, they recommend that poor responders to neoadjuvant CAB should be treated with more intensive therapy using CAB combined with high dose rate (HDR)-brachytherapy, intensity-moderated radiotherapy (I-MRT), or some forms of chemotherapy.
QOL and medical cost
Long-term hormonal therapy is sometimes criticized for reducing patients’ QOL. In our institution, the QOL of prostate cancer patients treated with primary hormonal therapy was investigated using the Androgen Deficiency in Aging Male (ADAM) questionnaire to allow comparison with healthy aged men who visited our institution for medical examinations. Surprisingly, the QOL of men receiving primary hormonal therapy was rather better than that of the healthy controls, except for sexual function in men aged 50-59 years (57). Indeed, most prostate cancer patients reported no anxiety regarding their primary disease or side effects of the treatment. Kato et al. evaluated health-related QOL (HRQOL) in Japanese men receiving hormonal therapy for prostate cancer using SF-36 and USLA-PCI59). They concluded that general HRQOL was mostly unaffected by hormonal therapy and that most patients did not report sexual anxiety despite deterioration of sexual function. These reports suggest that QOL of prostatic cancer patients receiving hormonal therapy is rather better than previously thought, at least in Japan (58).
Medical costs can also be a significant issue. The medical costs of hormonal therapy are higher than those of other treatments, but there are costs that are calculated directly, such as medical costs or transportation for hospital visits, and costs that cannot be calculated, such as loss of employment for disease treatment or psychological burden. Therefore, estimation of cost is very difficult, and further studies are required to compare costs with other types of treatment.
Adverse effects
Several recent studies indicated that ADT increases the incidences of cardiovascular disease and bone fractures. Keating et al. demonstrated that GnRH agonist increased the risk of diabetes mellitus (DM), coronary heart disease (CHD), myocardial infarction, and sudden cardiac death compared with the risks in patients without hormonal therapy (59). However, their study had some limitations. First, this was not a randomized study. Therefore, patients receiving GnRH agonist may have been associated with higher levels of background factors contributing to DM or heart disease. For example, older men who are more likely to receive hormonal therapy are also more likely to develop DM or CHD. Second, we cannot exclude the possibility that men receiving regular injections were more likely to be diagnosed with DM or CHD because of the greater frequency of medical consultations. D’Amico et al. showed that a subset of men age 65 years or older who received 6 months of ADT demonstrated shorter intervals to fatal myocardial infarction compared with men in this age group who did not receive ADT (60). However, this study was criticized by the authors of another paper recently published in the same journal (61). One major criticism was that D’Amico et al. did not show any difference in total number of fatal myocardial infarctions between groups. Their study was also criticized for its short treatment duration, shorter follow-up, and the lack of information on cardiovascular disease (CVD) risk factors. Efstatiou et al. described the first analysis using data from a large prospective study to directly address the potential relationship between GnRH agonists and cardiovascular mortality (61). In this study, patients with locally advanced prostate cancer who selected radiotherapy were randomly assigned to one of two arms. Patients in arm 1 received radiotherapy plus adjuvant hormonal therapy for 4.2 years on average. Those in arm 2 initially received only radiotherapy, and thereafter 64% of patients received salvage hormonal therapy after recurrence. Pretreatment characteristics, including CVD risk factors, were similar between the two arms. Surprisingly, at 9 years, cardiovascular mortality rate for men treated with adjuvant hormonal therapy was 8.4%, which was less than the rate of 11.4% for men without adjuvant hormonal therapy. However, patients with established CVD risk factors were significantly associated with greater cardiovascular mortality. Therefore, criticism of hormonal therapy should not be simplistic, but rather should focus on decreasing cardiovascular risk factors and managing CVD.
With regard to the adverse effects of hormonal therapy data for the general population show that the incidence of ischemic heart disease is much lower in Japanese than in Westerner subjects. The incidence of bone fractures is much lower in Japanese than in Western populations. Based on these data, we expect that the adverse effects of hormonal therapy will be less in Japanese populations. Akaza et al. conducted the J CaP study as a surveillance study of hormonal therapy in Japan (62). The data showed that the cardiovascular mortality rate in Japanese patients undergoing ADT was almost the same as the rate in the general population, as expected. Nevertheless, androgen deprivation could induce a variety of adverse effects even in Japan, because the adoption of a more Western lifestyle may increase the susceptibility to adverse effects of hormonal therapy. Therefore, efforts should be made to prevent or minimize such adverse effects. Management strategies for ADT-associated morbidities are shown in Table 1. It is well known that bone mineral density is decreased during long-term ADT. Therefore, the fracture rate after ADT is not low. We performed a nonrandomized prospective study to confirm the usefulness of bisphosphonate for improvement of bone mineral density in patients receiving hormonal therapy (63). Whereas bone mineral densities of patients not receiving risedronate continued to decrease, those of patients receiving risedronate increased. Management of endocrine and metabolic dysfunctions, such as DM, is very important, although most urologists do not pay adequate attention to such nonsurgical issues. Androgen deficiency is now attracting attention as one of the causes of metabolic syndrome. Basaria et al. reported that hormonal therapy induces metabolic syndrome, which they detected in more than 50% of men receiving long-term ADT (64). Therefore, we should carefully manage patients receiving hormonal therapy, and this is not as difficult as performing complicated surgery. The Endocrine Society Clinical Practice Guidelines will be helpful in preventing cardiovascular disease and DM. Furthermore, we should make our own clinical guidelines for urologists managing prostate cancer patients with hormonal therapy.

Full table
Patient satisfaction
The Prostate Cancer Outcome Study yielded interesting results (65). In this study, patient satisfaction was compared after each treatment, i.e., watchful waiting, primary androgen deprivation, radiotherapy, and radical prostatectomy. Satisfaction was higher in men receiving primary ADT than in those managed by watchful waiting or radical prostatectomy. In addition, most patients indicated that they would make the same choice if they had to select the treatment again. Thus, in patients requiring hormonal therapy, criticism of primary ADT should not be simplistic, but rather efforts should focus on decreasing its adverse effects.
Conclusions
Since its introduction about 70 years ago by Huggins and Hodges (1), hormonal therapy has played as important role in the treatment of prostate cancer. Recently, however, hormonal therapy has been the subject of frequent criticism. Some authors reported that it showed minimal effectiveness, while others suggested that it may reduce patients’ QOL and induce adverse effects. Such reports should be evaluated very carefully (66). From Japanese experiences using hormonal therapy, we suspect that there may be ethnic differences in efficacy and adverse effects of hormonal therapy. Therefore, it is necessary to accumulate further clinical evidence concerning the efficacy and adverse effects of hormonal therapy. We should also strive to decrease its adverse effects, because changes in lifestyle may increase susceptibility to the adverse effects of hormonal therapy even in Japan. It is expected that new hormonal compounds, such as selective androgen receptor modulators [SARM (67)] capable of specifically targeting prostate cancer, will be developed in the near future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Huggins C, Hodges C. Studies on prostate cancer.1. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941;1:293-7.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008;300:173-81. [PubMed]
- Green HJ, Pakenham KI, Headley BC, et al. Coping and health-related quality of life in men with prostate cancer randomly assigned to hormonalmedication of close monitoring. Psychooncology 2002;11:401-14. [PubMed]
- Grant JD, Litwin MS, Lee SP, et al. Does hormone therapy exacerbate the adverse effects of radiotherapy in men with prostate cancer? A quality of life study. J Urol 2011;185:1674-80. [PubMed]
- Labrie F, Luu-The V, Liu SX, et al. The key role of 17β-HSDs in sex steroid biology. Steroids 1997;62:148-58. [PubMed]
- Mizokami A, Koh E, Fujita H, et al. The adrenal androgen, androstenediol, is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen recetor. Cancer Res 2004;64:765-71. [PubMed]
- Narimoto K, Mizokami A, Izumi K, et al. Adrenal androgen levels as predictors of outcome in castration-resistant prostate cancer patients treated with combined androgen blockade using flutamide as a second-line anti-androgen. Int J Urol 2010;17:337-45. [PubMed]
- Akaza H, Hinotsu S, Usami M. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009;115:3437-45. [PubMed]
- Suzuki H, Okihara K, Miyake H. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol 2008;180:921-7. [PubMed]
- Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:12-23. [PubMed]
- Scholz M, Jennrich R, Strum S, et al. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J Urol 2005;173:1947-52. [PubMed]
- Izumi K, Kadono Y, Shima T, et al. Ethinylestradiol improves prostate-specific antigen levels in pretreated castration-resistant prostate cancer patients. Anticancer Res 2010;30:5201-5. [PubMed]
- Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815-25. [PubMed]
- Namiki M, Kitamura M, Buczko E, et al. Rat testis P-450(17)alpha cDNA: the deduced amino acid sequence, expression and secondary structural configuration. Biochem Biophys Res Commun 1988;157:705-12. [PubMed]
- Kitamura M, Buczko E, Dufau ML. Dissociation of hydroxylase and lyase activities by site-directed mutagenesis of the rat P45017 alpha. Mol Endocrinol 1991;5:1373-80. [PubMed]
- Koh Y, Buczko E, Dufau ML. Requirement of phenylalanine 343 for the preferential Δ4-lyase versus Δ5-lyase activity of rat CYP17. J Biol Chem 1993;268:18267-71. [PubMed]
- Attard G, Reid AHM, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 2009;27:3742-8. [PubMed]
- Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone in patients in patients with docetaxel treated castration-resistant prostate cancer. J Clin Oncol 2010;28:1496-501. [PubMed]
- Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res 2010;16:4319-24. [PubMed]
- Soifer HS, Souleimanian N, Wu S, et al. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J Biol Chem 2012;287:3777-87. [PubMed]
- Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787. [PubMed]
- Scher HI, Beer TM, Higano CS, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437. [PubMed]
- Sun S, Sprenger CCT, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest 2010;120:2715-30. [PubMed]
- Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. PNAS 2010;107:16759-65. [PubMed]
- Yamashita S, Lai K, Chuang K, et al. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia 2012;14:74-83. [PubMed]
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomized phase III trial. Lancet 2009;373:301-08. [PubMed]
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [PubMed]
- Bastian PJ, Gonzalgo ML, Aronson WJ, et al. Clinical and pathologic outcome after radical prostatectomy for prostate cancer patients with a preoperative Gleason sum of 8 to 10. Cancer 2006;107:1265-72. [PubMed]
- Zietman AL, Shipley WU. Androgen deprivation and radiation therapy in prostate cancer: the evolving case for combination therapy. Int J Radiat Oncol Biol Phys 1997;37:245-6. [PubMed]
- Joon DL, Hasegawa M, Sikes C, et al. Supraadditive apoptotic response of R3327-G rat prostate tumors to androgen ablation and radiation. Int J Radiat Oncol Biol Phys 1997;38:1071-7. [PubMed]
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360:103-6. [PubMed]
- D’Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004;292:821-7. [PubMed]
- Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;50:1243-52. [PubMed]
- Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516-27. [PubMed]
- Souhami L, Bae K, Pilepich M, et al. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol 2009;27:2137-43. [PubMed]
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26:2497-504. [PubMed]
- Stock RG, Cahlon O, Cesaretti JA, et al. Combined modality treatment in the management of high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2004;59:1352-9. [PubMed]
- Nag S, Beyer D, Friedland J, et al. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 1999;44:789-99. [PubMed]
- Konaka H, Egawa S, Saito S, et al. Tri-modality therapy with I-125 brachytherapy, external beam radiation therapy, and short- or long-term hormonal therapy for high-risk localized prostate cancer (TRIP): study protcol for a phase III, multicenter, randomized, controlled trial. BMC Cancer 2012;12:110. [PubMed]
- Messing EM, Manola J, Yao J. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006;7:472-9. [PubMed]
- Bittner N, Merrick GS, Wallner KE, et al. Interstitial brachytherapy should be standard of care for treatment of high-risk prostate cancer. Oncology (Williston Park) 2008;22:995-1004; discussion 1006, 1011-7.
- Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 Study. J Clin Oncol 2011;29:2040-5. [PubMed]
- Cancer Registration Committee of the Japanese Urological Association. Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol 2005;12:46-61. [PubMed]
- Maeda O. Option and indication for early stage prostate cancer. Jap J Cancer Chemother 2003;30:26-31.
- Fukagai T, Namiki TS, Carlile RG, et al. Comparison of clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int 2006;97:1190-3. [PubMed]
- Akaza H, Homma Y, Usami M, et al. Prostate Cancer Study Group. Efficacy of primary hormonal therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int 2006;98:573-9. [PubMed]
- Egawa M, Misaki T, Imao T, et al. Retrospective study on stage B prostate cancer in the Hokuriku District, Japan. Int J Urol 2004;11:304-9. [PubMed]
- Kitagawa Y, Koshida K, Mizokami A, et al. Pathological effects of neoadjuvant hormonal therapy help predict progression of prostate cancer after radical prostatectomy. Int J Urol 2003;10:377-82. [PubMed]
- Schulman CC, Debruyne FM, Forster G, et al. 4-year-follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. Eur Urol 2000;38:706-13. [PubMed]
- Labrie F, Candas B, Gomez JL, et al. Can combined androgen blockade provide long-term control or piossible cure of localized prostate cancer? Urology 2002;60:115-19. [PubMed]
- Ueno S, Namiki M, Fukagai T, et al. Efficacy of primary hormonal therapy for patients with localized and locally advanced prostate cancer: a retrospective multicenter study. Int J Urol 2006;13:1494-500. [PubMed]
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [PubMed]
- Labrie F, Bélanger A, Luu-The V, et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev 2005;26:361-79. [PubMed]
- Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst 2003;95:981-9. [PubMed]
- Tanaka N, Hara H, Yamabe F, et al. Investigation on prostate re-biopsy and high-sensitivity PSA of prostate cancer patients receiving endocrine therapy. Jap J Urol 2005;96:196.
- Akakura K, Ito H, Sato N. Intermittent androgen suppression for prostate cancer. Nippon Rinsho 2000;58:289-91. [PubMed]
- Mizokami A, Ueno S, Fukagai T, et al. Global update on defining and treating high-risk localizing prostate cancer with leuprolin: an Asian perspective. BJU Int 2007;99:6-9. [PubMed]
- Kato T, Komiya A, Suzuki H, et al. Effect of androgen deprivation therapy on quality of life in Japanese men with prostate cancer. Int J Urol 2007;14:416-21. [PubMed]
- Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448-56. [PubMed]
- D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 2007;25:2420-5. [PubMed]
- Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG85-01. J Clin Oncol 2009;27:92-9. [PubMed]
- Akaza H. Future prospects for luteinizing-hormone-releasing hormone analogues in prostate cancer treatment. Pharmacology 2010;85:110-20. [PubMed]
- Izumi K, Mizokami A, Sugimoto K, et al. Risedronate recovers bone loss in patients with prostate cancer undergoing androgen-deprivation therapy. Urology 2009;73:1342-6. [PubMed]
- Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 2006;106:581-8. [PubMed]
- Hoffman RM, Hunt WC, Gilliland FD, et al. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma. Results from the Prostate Cancer Outcomes Study. Cancer 2003;97:1653-62. [PubMed]
- Namiki M, Mizokami A, Akaza H. Should patients with localized prostate cancer receive primary androgen deprivation therapy? Nat Clin Pract Urol 2008;5:648-9. [PubMed]
- Min L, Yanase T, Tanaka T, et al. A novel synthetic androgen receptor ligand, S42, works as a selective androgen receptor modulator and possesses metabolic effects with little impact on the prostate. Endocrinology 2009;150:5606-16. [PubMed]